Adakveo Injection: Package Insert / Prescribing Info
Package insert / product label
Generic name: crizanlizumab
Dosage form: injection
Drug class: Miscellaneous uncategorized agents
J Code (medical billing code): J0791 (5 mg, injection)
Medically reviewed by Drugs.com. Last updated on Jun 8, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
ADAKVEO® (crizanlizumab-tmca) injection, for intravenous use
Initial U.S. Approval: 2019
Recent Major Changes
Indications and Usage for Adakveo Injection
ADAKVEO is a selectin blocker indicated to reduce the frequency of vasoocclusive crises in adults and pediatric patients aged 16 years and older with sickle cell disease. (1)
Adakveo Injection Dosage and Administration
Dosage Forms and Strengths
Injection: 100 mg/10 mL (10 mg/mL) solution in a single-dose vial. (3)
Contraindications
None. (4)
Warnings and Precautions
- Infusion-Related Reactions: Monitor for and advise patients of signs and symptoms. Discontinue ADAKVEO infusion for severe reactions and manage medically. Temporarily interrupt or slow the rate of infusion for mild or moderate infusion-related reactions and initiate symptomatic treatment. Exercise caution with corticosteroids in patients with sickle cell disease unless clinically indicated (e.g., treatment of anaphylaxis). (5.1)
- Interference With Automated Platelet Counts (platelet clumping): Run test as soon as possible or use citrate tubes. (5.2)
Adverse Reactions/Side Effects
The most common adverse reactions (incidence ≥ 10%) were headache, arthralgia, nausea, back pain, fatigue, abdominal pain, pyrexia, diarrhea, vomiting, and oropharyngeal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Pregnancy: May cause fetal harm. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2024
Full Prescribing Information
1. Indications and Usage for Adakveo Injection
ADAKVEO® is indicated to reduce the frequency of vaso-occlusive crises (VOCs) in adults and pediatric patients aged 16 years and older with sickle cell disease.
2. Adakveo Injection Dosage and Administration
2.1 Recommended Dosage
Administer ADAKVEO 5 mg/kg by intravenous infusion over a period of 30 minutes at Week 0, Week 2, and every 4 weeks thereafter.
If a dose is missed, administer ADAKVEO as soon as possible.
If ADAKVEO is administered within 2 weeks after the missed dose, continue dosing according to the patient's original schedule.
If ADAKVEO is administered more than 2 weeks after the missed dose, continue dosing every 4 weeks thereafter.
Physicians should reevaluate the treatment at least yearly, to assess individual patient’s response to treatment and consider discontinuing therapy with ADAKVEO if no perceived benefit is achieved.
ADAKVEO may be given with or without hydroxyurea.
2.2 Preparation and Administration
ADAKVEO should be prepared and administered by a healthcare professional.
Preparation
- Use aseptic technique to prepare the solution for infusion.
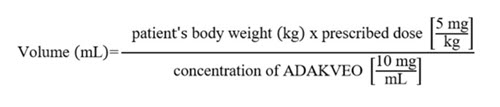
- Calculate the dose (mg) and the total volume (mL) of ADAKVEO solution required, and the number of ADAKVEO vials needed based on the patient’s actual body weight.
- Prepare 5 mg of ADAKVEO per kg of actual body weight.
- Calculate the volume of ADAKVEO to be used according to the following equation:
Dilution
Dilute ADAKVEO in 0.9% Sodium Chloride Injection or 5% Dextrose Injection to a total volume of 100 mL for intravenous infusion as follows:
- Obtain the number of vials required. One vial is needed for every 10 mL of ADAKVEO.
- Bring vials to room temperature for a maximum of 4 hours prior to the start of preparation (piercing the first vial).
- Visually inspect the vials.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- ADAKVEO is clear to opalescent, colorless or may have a slightly brownish-yellow tint.
- Do not use if particles are present in the solution.
- Obtain a 100 mL 0.9% Sodium Chloride Injection or 5% Dextrose Injection infusion bag/container.
- Infusion bags/containers must be made of either polyvinyl chloride (PVC), polyethylene (PE), or polypropylene (PP).
- Remove a volume of 0.9% Sodium Chloride Injection or 5% Dextrose Injection from the infusion bag/container that is equal to the required volume of ADAKVEO solution.
- Withdraw the necessary amount of ADAKVEO solution and dilute by adding to the infusion bag/container containing 0.9% Sodium Chloride Injection or 5% Dextrose Injection.
- The volume of ADAKVEO added to the infusion bag/container should not exceed 96 mL.
- Gently invert the infusion bag to mix the diluted solution. DO NOT SHAKE.
- Single-dose vials. Discard unused portion.
Storage Conditions of the Diluted Solution
Administer ADAKVEO diluted solution as soon as possible. If not administered immediately, store the prepared solution either:
- At room temperature up to 25°C (77°F) for no more than 4.5 hours from the start of the preparation (piercing the first vial) to the completion of infusion.
- Under refrigeration at 2°C to 8°C (36°F to 46°F) for no more than 24 hours, from the start of the time of the preparation (piercing the first vial) to the completion of infusion. This includes the storage of the diluted solution and the time to warm up to room temperature. Protect the diluted solution from light during storage under refrigeration.
Administration
- Administer ADAKVEO diluted solution by intravenous infusion over a period of 30 minutes through an intravenous line, which must contain a sterile, nonpyrogenic 0.2-micron inline filter.
- No incompatibilities have been observed between ADAKVEO and infusion sets composed of PVC, polyethylene (PE-lined PVC), polyurethane (PU), and in-line filter membranes composed of polyethersulfone (PES, neutral and positively charged), positively charged polyamide (PA), and polysulphone (PSU).
- Do not mix or coadminister with other drugs through the same intravenous line.
- After administration of ADAKVEO, flush the line with at least 25 mL of 0.9% Sodium Chloride Injection or 5% Dextrose Injection.
- Dispose of any unused product or waste material in accordance with local requirements.
3. Dosage Forms and Strengths
Injection: 100 mg/10 mL (10 mg/mL) as a clear to opalescent, colorless to slightly brownish-yellow solution in a single-dose vial.
5. Warnings and Precautions
5.1 Infusion-Related Reactions
In the SUSTAIN clinical trial, infusion-related reactions (IRRs) (defined as occurring during/within 24 hours of infusion) were observed in 2 (3%) patients treated with ADAKVEO 5 mg/kg. In the STAND clinical trial, IRRs were observed in 6 (7%) patients treated with ADAKVEO 5 mg/kg.
IRRs presented most frequently as pain, nausea, vomiting, fatigue, dizziness, pruritis, diarrhea, and pyrexia. Some IRRs have required hospitalizations. The majority of these IRRs occurred during the first and second infusions.
Monitor for and advise patients to report signs and symptoms of IRRs. Discontinue ADAKVEO infusion for severe IRRs and institute appropriate medical care. Consider permanent discontinuation of ADAKVEO treatment in case of severe IRRs. Manage mild to moderate infusion-related reactions with temporary interruption of infusion, slowing the rate of infusion, symptomatic treatment (e.g., acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDS), opioids, antihistamines, intravenous fluids, and/or oxygen therapy). For subsequent infusions, consider premedication and/or infusion rate reduction.
Exercise caution with corticosteroids in patients with sickle cell disease unless clinically indicated (e.g., treatment of anaphylaxis). Use of corticosteroids may increase the risk of complications such as acute chest syndrome and fat embolism.
Infusion-Related Reactions and Vaso-occlusive Crises
Infusion-related reactions are sometimes indistinguishable from vaso-occlusive crisis (VOC) events. IRRs and VOCs may occur concomitantly and/or VOCs may occur as a consequence of an IRR.
5.2 Laboratory Test Interference
Interference with automated platelet counts (platelet clumping) has been observed following administration of ADAKVEO, in particular, when blood samples were collected in tubes containing ethylenediaminetetraacetic acid (EDTA). Mitigation strategies are recommended [see Drug Interactions (7.1)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Infusion-related reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Sickle Cell Disease
SUSTAIN Trial
The safety of ADAKVEO was evaluated in the SUSTAIN trial [see Clinical Studies (14.1)]. Eligible patients were diagnosed with sickle cell disease (any genotype, including HbSS, HbSC, HbS beta0-thalassemia, HbS beta+-thalassemia, and others). Patients received ADAKVEO 5 mg/kg (N = 66) or 2.5 mg/kg (N = 64) or placebo (N = 62) administered by intravenous infusion on Week 0, Week 2, and every 4 weeks thereafter. The safety evaluation below is limited to the patients who received the recommended dose of 5 mg/kg.
Among the 66 patients that received the recommended dose (5 mg/kg), 83% were exposed for 6 months or longer and 61% were exposed for approximately one year; forty-two (64%) patients were treated with ADAKVEO in combination with hydroxyurea.
Serious adverse reactions were reported in 2 patients (3%) treated with ADAKVEO 5 mg/kg; both reactions were pyrexia.
Two deaths (3%) occurred in the ADAKVEO 5 mg/kg treatment group. None of the deaths were considered to be related to ADAKVEO.
The most common adverse reactions (≥ 10%) were arthralgia, nausea, back pain, abdominal pain, pyrexia, and diarrhea.
Table 1 summarizes the adverse reactions in the SUSTAIN trial.
| aAbdominal pain: abdominal pain, upper abdominal pain, lower abdominal pain, and abdominal tenderness. | ||||
| ADAKVEO 5 mg/kg N = 66 % | Placebo N = 62 % |
|||
| Adverse Reactions | All Grades % | Grade ≥ 3 % | All Grades % | Grade ≥ 3 % |
| Arthralgia | 18 | 2 | 8 | 2 |
| Nausea | 18 | 0 | 11 | 2 |
| Back pain | 15 | 0 | 11 | 0 |
| Abdominal paina | 12 | 0 | 5 | 0 |
| Pyrexia | 11 | 12 | 7 | 0 |
| Diarrhea | 11 | 0 | 3 | 2 |
Clinically relevant adverse reactions (all Grades) that were reported in less than 10% of patients treated with ADAKVEO included: oropharyngeal pain, vomiting, pruritus (pruritus and vulvovaginal pruritus), musculoskeletal chest pain, myalgia, infusion-site reaction (infusion-site extravasation, infusion-site pain, and infusion-site swelling), and infusion-related reaction.
STAND Trial
The safety of ADAKVEO was also evaluated in the STAND trial [see Clinical Studies (14.1)]. Eligible patients were diagnosed with sickle cell disease (any genotype, including HbSS, HbSC, HbS beta0-thalassemia, HbS beta+-thalassemia, and others). Patients received ADAKVEO 5 mg/kg (N = 84) or 7.5 mg/kg (N = 83) or placebo (N = 85) administered by intravenous infusion on Week 0, Week 2, and every 4 weeks thereafter. The safety evaluation below is limited to the patients who received the recommended dose of 5 mg/kg.
Among the 84 patients that received the recommended dose (5 mg/kg), 93% were exposed for approximately 6 months or longer and 88% were exposed for approximately one year; sixty-two (74%) patients were treated with ADAKVEO in combination with hydroxyurea.
Serious adverse reactions were reported in 2 patients (2%) treated with ADAKVEO 5 mg/kg and this reaction was pain. The most common adverse reactions (≥ 10%) were headache, nausea, fatigue, vomiting, and oropharyngeal pain.
| aFatigue includes asthenia and malaise. | ||||
| ADAKVEO 5 mg/kg N = 84 % | Placebo N = 85 % |
|||
| Adverse Reactions | All Grades % | Grade ≥ 3 % | All Grades % | Grade ≥ 3 % |
| Headache | 25 | 1 | 19 | 0 |
| Nausea | 17 | 0 | 9 | 0 |
| Fatiguea | 13 | 0 | 8 | 0 |
| Vomiting | 10 | 0 | 5 | 0 |
| Oropharyngeal pain | 10 | 0 | 4 | 0 |
Clinically relevant adverse reactions (all Grades) that were reported in less than 10% of patients treated with ADAKVEO or events having a difference of less than 3% between ADAKVEO treatment arms and placebo included: diarrhea, pruritus, dizziness, infusion-related reaction, infusion-site reaction.
Related/similar drugs
7. Drug Interactions
7.1 Laboratory Test Interference
Platelet Tests
ADAKVEO interferes with automated platelet counts (platelet clumping) in particular when blood samples are collected in tubes containing EDTA, which may lead to unevaluable or falsely decreased platelet counts. Run blood samples within 4 hours of blood collection or collect blood samples in tubes containing citrate. When needed, estimate platelet count via peripheral blood smear.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on data from animal studies, ADAKVEO has the potential to cause fetal harm when administered to a pregnant woman. In an animal reproduction study, intravenous administration of crizanlizumab-tmca to pregnant cynomolgus monkeys from the onset of organogenesis through delivery resulted in a non-dose related increased fetal loss (abortions/stillbirths) at doses approximately 2.8 times the exposure at the recommended clinical dose at 5 mg/kg/dose once every 4 weeks (see Data).
There are insufficient human data on ADAKVEO use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Advise pregnant women of the potential risk to a fetus. ADAKVEO should only be used during pregnancy if the expected benefit to the patient justifies the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is approximately 14% and up to 43%, respectively. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Women with sickle cell disease have an increased risk of adverse pregnancy outcomes for the mother and the fetus. Pregnant women are at greater risk for VOCs, pre-eclampsia, eclampsia, and maternal mortality. For the fetus, there is an increased risk for intrauterine growth restriction, preterm delivery, low birth weight, and perinatal mortality.
Data
Animal Data
In an enhanced pre- and postnatal development study in cynomolgus monkeys, pregnant animals received intravenous doses of crizanlizumab-tmca at 10 and 50 mg/kg once every 2 weeks during the period of onset of organogenesis through delivery. No maternal toxicity was observed. Maternal exposures at doses of 10 and 50 mg/kg were between 2.8 and 16 times, respectively, the human clinical exposure based on area under the curve (AUC) in patients with sickle cell disease at 5 mg/kg/dose once every 4 weeks. There was an increase in fetal loss (abortions or still births) at both crizanlizumab-tmca doses which was higher in the third trimester.
There were no effects on infant growth and development through 6-months postpartum that were attributable to crizanlizumab-tmca.
Measurable crizanlizumab-tmca serum concentrations were observed in the infant monkeys at postnatal Day 28, confirming that crizanlizumab crosses the placental barrier.
8.2 Lactation
Risk Summary
There is no data on the presence of crizanlizumab-tmca in human or animal milk, the effects on the breastfed child, or the effects on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to crizanlizumab-tmca are unknown.
The developmental and health benefits of breast-feeding should be considered along with the mother’s clinical need for ADAKVEO and any potential adverse effects on the breastfed child from ADAKVEO or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of ADAKVEO for sickle cell disease have been established in pediatric patients aged 16 years and older. Use of ADAKVEO for sickle cell disease is supported by evidence from adequate and well-controlled studies in adults and pediatric patients (SUSTAIN trial). The SUSTAIN trial enrolled one pediatric patient treated with ADAKVEO 5 mg/kg aged 16 years old [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), Clinical Studies (14)].
The safety and efficacy of ADAKVEO in pediatric patients below the age of 16 years have not been established.
8.5 Geriatric Use
There were no ADAKVEO-treated patients 65 years of age and older in the clinical studies for sickle cell disease [see Clinical Studies (14)]. Clinical studies of ADAKVEO did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
11. Adakveo Injection Description
Crizanlizumab-tmca is a P-selectin blocker humanized IgG2 kappa monoclonal antibody that binds to P-selectin. Crizanlizumab-tmca is produced using recombinant DNA technology in Chinese hamster ovary (CHO) cells. It is composed of 2 heavy chains, each containing 448 amino acids, and 2 light chains each containing 218 amino acids, with a theoretical molecular weight of approximately 146 kDa.
ADAKVEO (crizanlizumab-tmca) injection is supplied as a sterile, preservative-free, clear to opalescent, colorless to slightly brownish-yellow solution for dilution and subsequent administration by intravenous infusion. Each 10 mL vial contains 100 mg crizanlizumab-tmca, citric acid (5.4 mg), polysorbate 80 (2 mg), sodium citrate (50.5 mg), sucrose (753.3 mg), and water for injection with a pH of 6.
12. Adakveo Injection - Clinical Pharmacology
12.1 Mechanism of Action
Crizanlizumab-tmca is a humanized IgG2 kappa monoclonal antibody that binds to P-selectin and blocks interactions with its ligands, including P-selectin glycoprotein ligand 1 (PSGL-1). Crizanlizumab can also dissociate preformed P-selectin/PSGL-1 complex.
Binding P-selectin on the surface of the activated endothelium and platelets blocks interactions between endothelial cells, platelets, red blood cells, and leukocytes.
12.2 Pharmacodynamics
ADAKVEO resulted in a dose-dependent P-selectin inhibition (measured ex vivo) in patients with sickle cell disease and healthy volunteers.
12.3 Pharmacokinetics
The pharmacokinetics of crizanlizumab-tmca were evaluated in healthy volunteers and patients with sickle cell disease. The mean crizanlizumab-tmca Cmax, AUClast, or AUCinf increased disproportionally over the dose range of 0.2 to 8 mg/kg (0.04 to 1.6 times the approved recommended dosage) in healthy volunteers. In healthy volunteers administered the 5 mg/kg dose, the mean [coefficient of variation (CV%)] crizanlizumab-tmca Cmax, AUClast, or AUCinf were 0.16 (15.3%) mg/mL, 33.6 (12.6%) mg*hr/mL and 34.6 (13.1%) mg*hr/mL, respectively.
Distribution
The mean (% CV) volume of distribution was 4.26 (25.1%) L after a single crizanlizumab-tmca 5 mg/kg intravenous infusion in healthy volunteers.
Elimination
The mean (% CV) terminal elimination half-life (t1/2) of crizanlizumab-tmca was 10.6 (20.5%) days and the mean clearance was 11.7 (16.2%) mL/hr at 5 mg/kg doses in healthy volunteers. The mean (% CV) elimination t1/2 of crizanlizumab-tmca was 11.4 (31.5%) days during dosing interval in patients with sickle cell disease.
Metabolism
Crizanlizumab-tmca is expected to be metabolized into small peptides by catabolic pathways.
Specific Populations
The effect of renal or hepatic impairment on the pharmacokinetics of crizanlizumab-tmca is unknown.
Drug Interaction Studies
Hydroxyurea had no clinically meaningful effect on crizanlizumab-tmca pharmacokinetics in patients in clinical studies.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of ADAKVEO or of other crizanlizumab products.
The immunogenicity of ADAKVEO was evaluated using a validated bridging immunoassay for the detection of binding anti-crizanlizumab-tmca antibodies. In a single arm, open label multiple dose study, 0 of the 45 patients with sickle cell disease treated with ADAKVEO 5 mg/kg tested positive for treatment-induced anti-crizanlizumab-tmca antibodies. In a single-dose study in healthy subjects, 1 of the 61 (1.6%) evaluable subjects tested positive for a treatment-induced anti-crizanlizumab-tmca antibodies. No treatment induced anti-crizanlizumab-tmca antibodies were detected (0 of 84 patients) in a Phase 3 study at 5 mg/kg over the 52 week time period (samples collected at baseline, Weeks 3, 15, 19, 27, and 51). Therefore, no significant effect on pharmacokinetics or pharmacodynamics has been observed or is expected.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or genotoxicity studies have been conducted with crizanlizumab-tmca.
In the 26-week repeat-dose toxicity study, cynomolgus monkeys were administered crizanlizumab-tmca once every 4 weeks at doses up to 50 mg/kg (at least 13.1 times the human clinical exposure based on AUC in patients with sickle cell disease at 5 mg/kg once every 4 weeks). There were no adverse effects of crizanlizumab-tmca on male or female reproductive organs.
14. Clinical Studies
SUSTAIN
The efficacy of ADAKVEO was evaluated in patients with sickle cell disease in SUSTAIN [NCT01895361], a 52-week, randomized, multicenter, placebo-controlled, double-blind study. A total of 198 patients with sickle cell disease, any genotype (HbSS, HbSC, HbS/beta0-thalassemia, HbS/beta+-thalassemia, and others), and a history of 2-10 VOCs in the previous 12 months were eligible for inclusion. Patients were randomized 1:1:1 to ADAKVEO 5 mg/kg (N = 67), ADAKVEO 2.5 mg/kg (N = 66), or placebo (N = 65) administered over a period of 30 minutes by intravenous infusion on Week 0, Week 2, and every 4 weeks thereafter for a treatment duration of 52 weeks. Randomization was stratified by patients already receiving hydroxyurea (Y/N) and by the number of VOCs in the previous 12 months (2 to 4, 5 to 10).
Patients received ADAKVEO (with or without hydroxyurea) and were allowed to receive occasional transfusions and pain medications [i.e., acetaminophen, NSAIDs, and opioids] on an as needed basis.
Patients recruited in the study had complications associated with sickle cell disease and other comorbidities, including a history of acute chest syndrome (18%); pulmonary hypertension (8%); priapism (7%); psychiatric manifestations (25%), including depression and anxiety; hypertension (17%); cholelithiasis (17%). Demographic and other baseline characteristics were similar among the treatment groups (see Table 3).
| Abbreviation: VOCs, vasoocclusive crises. | ||
| ADAKVEO 5 mg/kg (N = 67) | Placebo (N = 65) |
|
| Age (years) | ||
| Median | 29 | 26 |
| Range | 16, 63 | 16, 56 |
| Gender, n (%) | ||
| Male | 32 (48%) | 27 (42%) |
| Female | 35 (52%) | 38 (59%) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 20 (30%) | 11 (17%) |
| Not Hispanic or Latino | 45 (67%) | 53 (82%) |
| Unknown | 2 (3%) | 1 (2%) |
| Race | ||
| Black or African American | 60 (90%) | 60 (92%) |
| White | 4 (6%) | 3 (5%) |
| Other | 3 (5%) | 2 (3%) |
| Sickle cell disease genotype, n (%) | ||
| HbSS | 47 (70%) | 47 (72%) |
| HbSC | 9 (13%) | 8 (12%) |
| HbS/beta0 - thalassemia | 3 (5%) | 7 (11%) |
| HbS/beta+ - thalassemia | 7 (10%) | 1 (2%) |
| Other | 1 (2%) | 2 (3%) |
| Hydroxyurea use, n (%) | ||
| Yes | 42 (63%) | 40 (62%) |
| No | 25 (37%) | 25 (39%) |
| Number of VOCs in previous 12 months, n (%) | ||
| 2 to 4 | 42 (63%) | 41 (63%) |
| 5 to 10 | 25 (37%) | 24 (37%) |
Efficacy was evaluated in the SUSTAIN study by the annual rate of VOCs leading to a healthcare visit. A VOC leading to a healthcare visit was defined as an acute episode of pain with no cause other than a vasoocclusive event that required a medical facility visit and treatment with oral or parenteral opioids, or parenteral NSAIDs. Acute chest syndrome, hepatic sequestration, splenic sequestration, and priapism (requiring a visit to a medical facility) were also considered VOCs.
Patients with sickle cell disease who received ADAKVEO 5 mg/kg had a lower median annual rate of VOC compared to patients who received placebo (1.63 vs. 2.98) which was statistically significant (p = 0.010). Reductions in the frequency of VOCs were observed among patients regardless of sickle cell disease genotype and/or hydroxyurea use.
Thirty-six percent (36%) of patients treated with ADAKVEO 5 mg/kg did not experience a VOC compared to 17% of placebo-treated patients. The median time to first VOC from randomization was 4.1 months in the ADAKVEO 5 mg/kg arm compared to 1.4 months in the placebo.
The main efficacy results of the pivotal study, SUSTAIN, are summarized in Table 4.
| Abbreviations: HL, hodges-lehmann; VOCs, vasoocclusive crises.
aVOCs were as assessed by an independent review committee. bStandard median. cHL median difference [95% confidence interval (CI)]. |
|||
| Event | ADAKVEO, 5 mg/kgb
(n = 67) | Placebob
(n = 65) | Treatment Difference Estimatec |
| Annual rate of VOCsa | 1.63 | 2.98 | HL = -1.01, (-2.00, 0.00) |
| Annual rate of days hospitalized | 4 | 6.87 | |
STAND
The efficacy of two doses of ADAKVEO, with or without HU/HC, was evaluated, but not established, in the STAND trial [NCT03814746], a randomized, placebo-controlled, double-blind, multicenter clinical study in adolescent and adult sickle cell disease patients with a history of VOCs. The efficacy results of STAND study are summarized in Table 6 below.
In this study, VOC was defined as a pain crisis (acute onset of pain for which there is no other medically determined explanation other than vaso-occlusion) which requires therapy with oral or parenteral opioids or parenteral NSAID. Acute chest syndrome (ACS), priapism and hepatic or splenic sequestration were considered VOCs in this study.
A total of 252 sickle cell disease patients were randomized to the study, 85 in placebo arm, 84 in ADAKVEO 5 mg/kg arm and 83 in ADAKVEO 7.5 mg/kg arm. The 7.5 mg/kg ADAKVEO dose is not approved and is not recommended for use. Demographic and other baseline characteristics were similar among the treatment groups (see Table 5).
| ADAKVEO 5 mg/kg (N = 84) | Placebo (N = 85) |
|
| Age (years) | ||
| Median | 24 | 25 |
| Range | 12, 64 | 12, 68 |
| Gender, n (%) | ||
| Female | 45 (54%) | 49 (58%) |
| Male | 39 (46%) | 36 (42%) |
| Race, n (%) | ||
| Black or African American | 46 (55%) | 43 (51%) |
| White | 27 (32%) | 26 (31%) |
| Asian | 6 (7%) | 6 (7%) |
| Other | 5 (6%) | 10 (12%) |
| Genotype, n (%) | ||
| HbSS | 58 (69%) | 58 (68%) |
| HbSC | 11 (13%) | 12 (14%) |
| HbS/beta+ - thalassemia | 8 (10%) | 8 (9%) |
| HbS/beta0 - thalassemia | 5 (6%) | 6 (7%) |
| Other | 2 (2%) | 1 (1%) |
| Ethnicity, n (%) | ||
| Not Hispanic or Latino | 54 (64%) | 57 (67%) |
| Hispanic or Latino | 22 (26%) | 18 (21%) |
| Other (not reported/unknown) | 8 (10%) | 10 (12%) |
| Hydroxyurea use, n (%) | ||
| Yes | 62 (74%) | 61 (72%) |
| No | 20 (24%) | 23 (27%) |
| Missing | 2 (2%) | 1 (1%) |
| Number of VOC leading to healthcare visit in the last 12 months, n (%) | ||
| < 5 | 62 (74%) | 58 (68%) |
| ≥ 5 | 21 (25%) | 27 (32%) |
| Missing | 1 (1%) | 0 |
The percentages for subgroups of race and genotype do not add up to 100% due to rounding to 1 decimal place. The results of the efficacy analysis did not confirm the statistical superiority of ADAKVEO over placebo in reducing VOCs leading to a healthcare visit over the first-year post randomization.
| n: Total number of participants included in the analysis. Obtained from fitting a negative binomial regression model with treatment and randomization stratification factors (baseline VOC and HU/HC) as covariates. The natural log of the observation period was used as offset. *The 95% CI includes 1, which indicates that the result is not statistically significant. |
||||||
| Between-Treatment Comparison | ||||||
| Treatment | n | Adjusted Annualized Rate of VOC | (95% CI) | Comparison | Rates Ratio | (95% CI) |
| ADAKVEO 5 mg/kg | 84 | 2.49 | (1.90, 3.26) | vs Placebo | 1.08 | (0.76, 1.55)* |
| Placebo | 85 | 2.30 | (1.75, 3.01) | |||
16. How is Adakveo Injection supplied
How Supplied
ADAKVEO (crizanlizumab-tmca) injection is a sterile, clear to opalescent, colorless to slightly brownish-yellow solution for intravenous infusion supplied as:
Carton containing one 100 mg/10 mL (10 mg/mL) single-dose vial NDC 0078-0883-61
The single-dose vial has a rubber stopper and an aluminum cap with a plastic flip-off disk. Each 10 mL vial is made of Type 1 glass.
Storage and Handling
- Store and transport refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light.
- Do not shake. Do not freeze.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Infusion-Related Reactions
Advise patients to contact their healthcare provider immediately for signs or symptoms of infusion-related reactions [see Warnings and Precautions (5.1)].
Interference With Automated Platelet Counts
Advise patients to inform their healthcare provider that they are receiving ADAKVEO prior to any blood tests due to the potential interference with laboratory tests used to measure platelet counts [see Warnings and Precautions (5.2)].
Manufactured by:
Novartis Pharmaceuticals Corporation
One Health Plaza
East Hanover, New Jersey 07936
US License No. 1244
© Novartis
T2024-48
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: 6/2024 |
| PATIENT INFORMATION | |
| ADAKVEO® (ah dak vee oh) (crizanlizumab-tmca) injection, for intravenous use |
|
| What is the most important information I should know about ADAKVEO? ADAKVEO may cause serious side effects, including: |
|
|
|
|
|
|
|
| What is ADAKVEO?
ADAKVEO is a prescription medicine used in people 16 years of age and older who have sickle cell disease to help reduce how often painful crises happen. It is not known if ADAKVEO is safe and effective in children under 16 years of age. |
|
Before receiving ADAKVEO, tell your healthcare provider about all of your medical conditions, including if you:
|
|
| How will I receive ADAKVEO? | |
|
|
| What are the possible side effects of ADAKVEO?
ADAKVEO may cause serious side effects.
|
|
|
|
|
These are not all of the possible side effects of ADAKVEO. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
| General information about the safe and effective use of ADAKVEO. | |
| Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about ADAKVEO that is written for health professionals. | |
| What are the ingredients in ADAKVEO? | |
| Active ingredient: crizanlizumab-tmca Inactive ingredients: citric acid, polysorbate 80, sodium citrate, sucrose, and water for injection |
|
| Manufactured by: Novartis Pharmaceuticals Corporation, One Health Plaza, East Hanover, New Jersey 07936 U.S. License No. 1244 © Novartis For more information, go to www.us.adakveo.com or call 1-888-NOWNOVA (1-888-669-6682). |
|
T2024-49
| ADAKVEO
crizanlizumab injection |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |
Biological Products Related to Adakveo
Find detailed information on biosimilars for this medication.
Frequently asked questions
More about Adakveo (crizanlizumab)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: miscellaneous uncategorized agents
- Breastfeeding
- En español