Coraspirina 81 mg Tabletas con cubierta enterica
Dosage form: tablet, coated
Ingredients: ASPIRIN 81mg
Labeler: Bayer HealthCare LLC.
NDC code: 0280-2055
Medically reviewed by Drugs.com. Last updated on Oct 25, 2024.
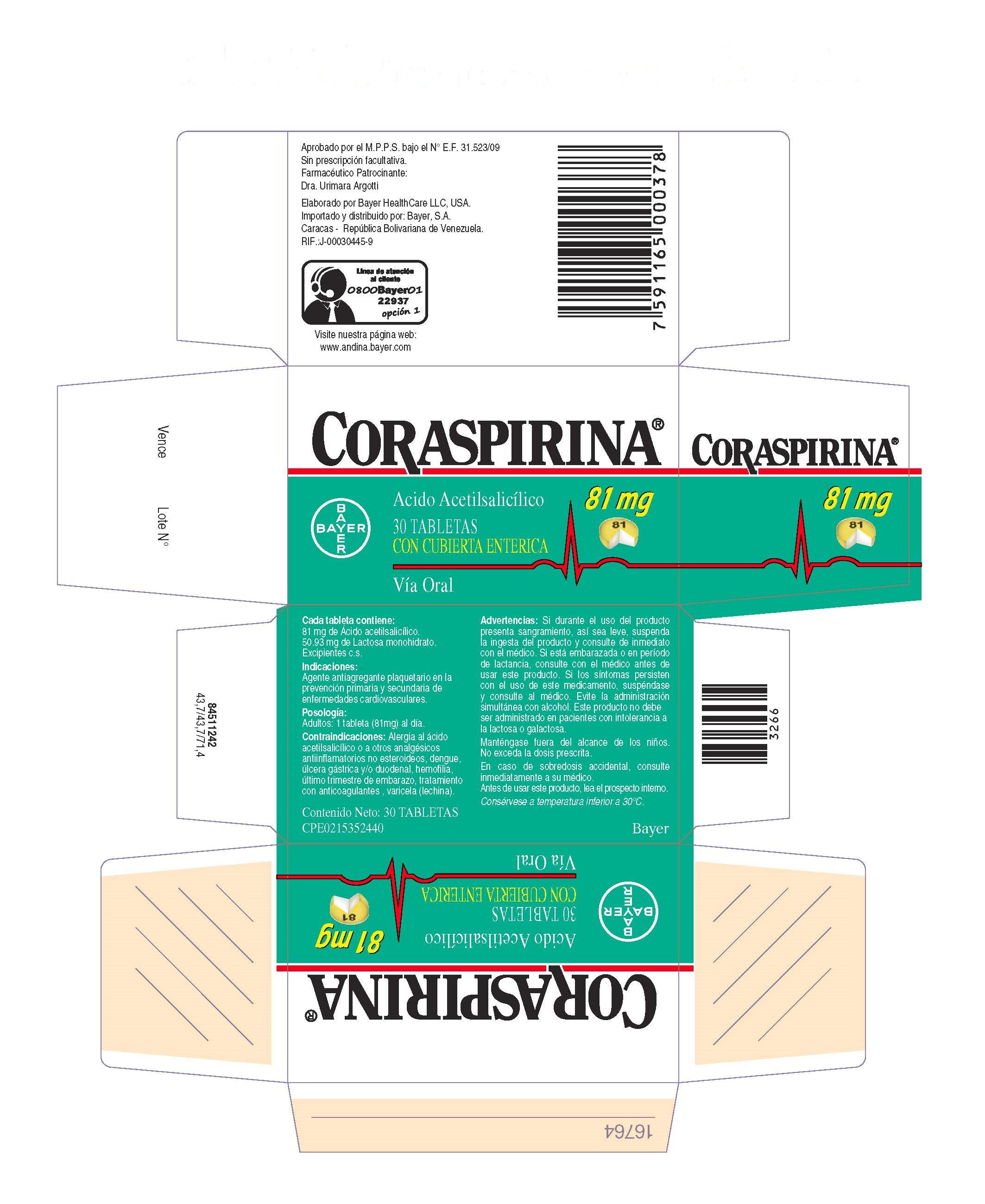
| CORASPIRINA 81 MG TABLETAS CON CUBIERTA ENTERICA
coraspirin 81 mg enteric coasted tablet tablet, coated |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
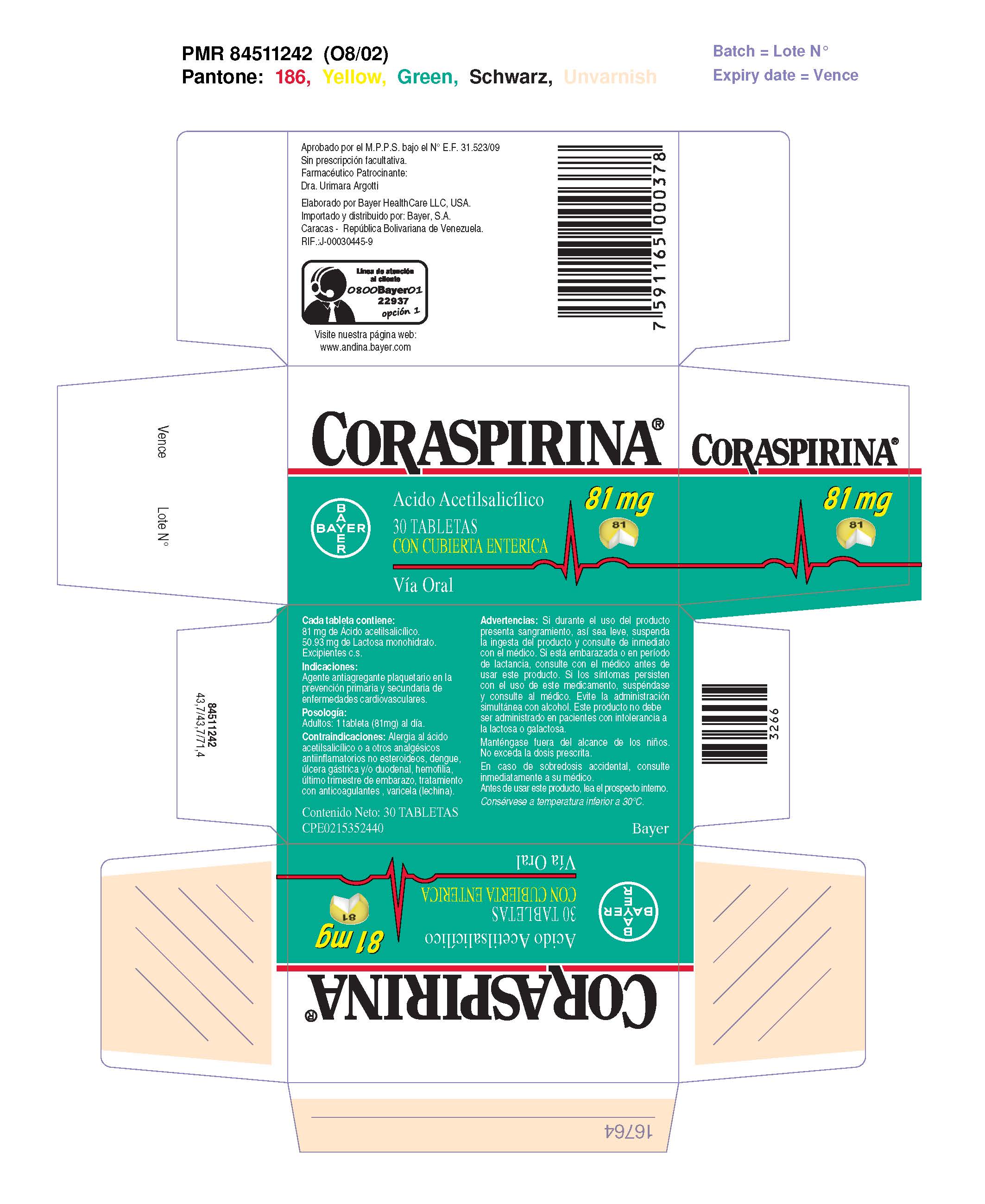 |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |
Document Id: 9689456f-cf98-3102-e053-2995a90acf2e
Set id: 255f8a0b-44e1-6b83-e054-00144ff8d46c
Version: 2
Bayer HealthCare LLC.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
