Dalay Maximum Strength
Dosage form: tablet
Ingredients: DIPHENHYDRAMINE HYDROCHLORIDE 25mg
Labeler: Genomma Labs USA, Inc
NDC code: 50066-247
Medically reviewed by Drugs.com. Last updated on Nov 6, 2024.
Diphenhydramine HCl 25 mg
Nighttime sleep aid
- for relief of occasional sleeplessness
- with any other products containing diphenhydramine, including one used on skin
- in children under 12 years of age
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
taking sedatives or tranquilizers
- marked drowsiness may occur
- avoid alcoholic beverages
- do not drive a motor vehicle or operate machinery
- sleeplessness persists continuously for more than 2 weeks.
Insomnia may be a symptom of serious underlying medical illness.
ask a health professional before use.
In case of accidental overdose, get medical help or contact a Poison Control Center (1-800-222-1222) immediately
Adults and children 12 years of age and older: take 2 tablets at bedtime if needed or as directed by a doctor
Children under 12 years of age: Do not use
- store at room temperature
- do not use if imprinted safety seal under cap is broken or missing
croscarmellose sodium, dicalcium phosphate dihydrate, FD&C blue #1 aluminum lake, magnesium stearate, microcrystalline cellulose, silicon dioxide
Call toll free 1-877-99 GENOM (43666)
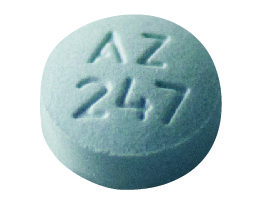
Dalay Sleep-Aid
| DALAY
MAXIMUM STRENGTH
diphenhydramine hcl 25 mg tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
 |
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Genomma Labs USA, Inc (832323534) |
| Registrant - Allegiant Health (079501930) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
