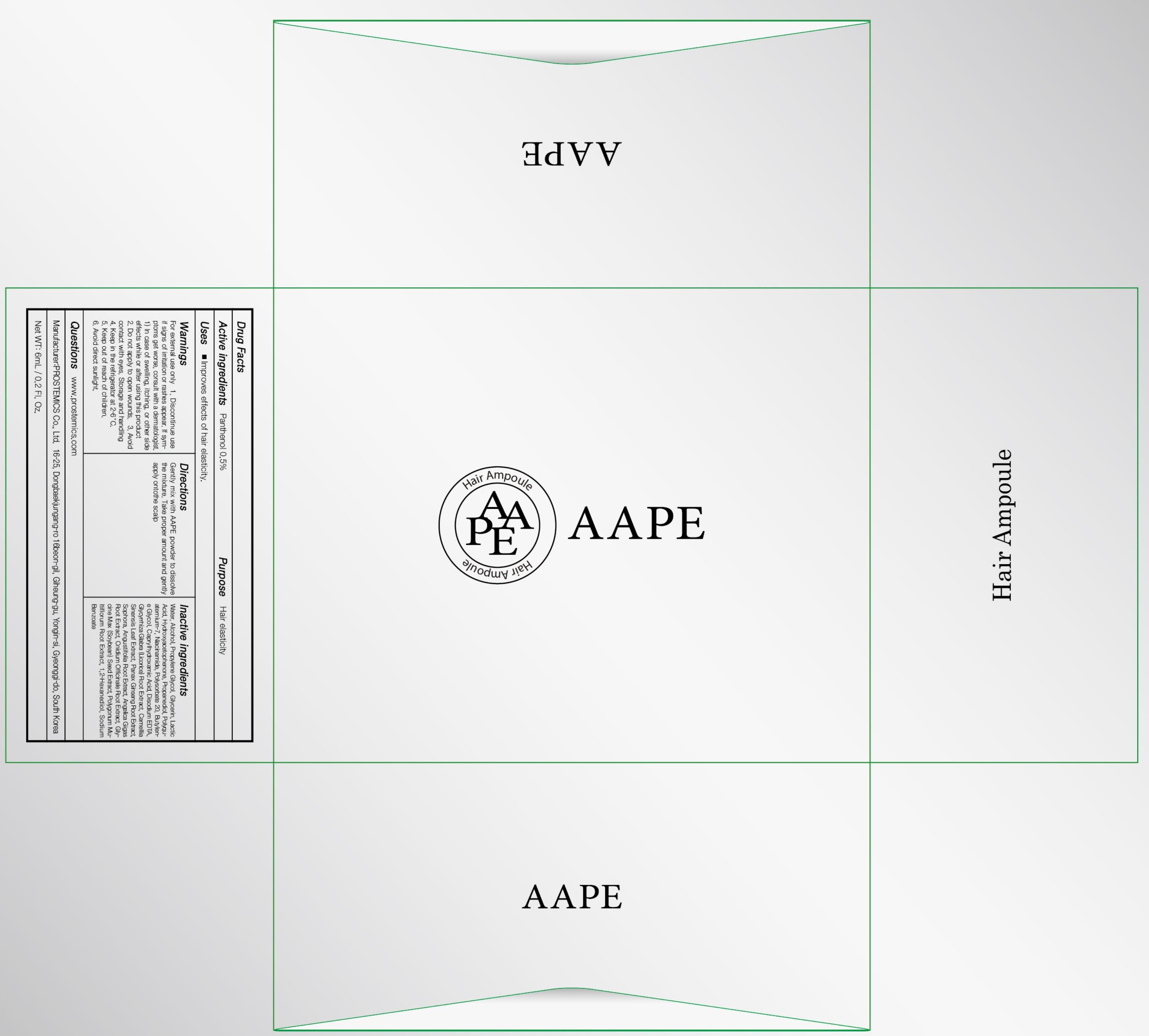
AAPE Hair Ampoule
Dosage form: liquid
Ingredients: Panthenol 0.03g in 6mL
Labeler: PROSTEMICS Co., Ltd.
NDC code: 62041-260
Medically reviewed by Drugs.com. Last updated on Aug 18, 2025.
Active ingredients: Panthenol 0.5%
Inactive ingredients:
Water, Alcohol, Propylene Glycol, Glycerin, Lactic Acid, Hydroxyacetophenone, Propanediol, Polyquaternium-7, Niacinamide, Polysorbate 20, Butylene Glycol, Caprylhydroxamic Acid, Disodium EDTA, Glycyrrhiza Glabra (Licorice) Root Extract, Camellia Sinensis Leaf Extract, Panax Ginseng Root Extract, Sophora, Angustifolia Root Extract, Angelica Gigas Root Extract, Cnidium Officinale Root Extract, Glycine Max (Soybean) Seed Extract, Polygonum Multiflorum Root Extract, 1,2-Hexanediol, Sodium Benzoate
Purpose: Hair elasticity
Warnings:
For external use only
1. Discontinue use if signs of irritation or rashes appear. If symptoms get worse, consult with a dermatologist. 1) In case of swelling, itching, or other side effects while or after using this product
2. Do not apply to open wounds.
3. Avoid contact with eyes.
Storage and handling
4. Keep in the refrigerator at 2-6°C.
5. Keep out of reach of children.
6. Avoid direct sunlight.
KEEP OUT OF REACH OF CHILDREN
Uses:
Improves effects of hair elasticity.
Directions:
Gently mix with AAPE powder to dissolve the mixture.
Take proper amount and gently apply onto the scalp.
| AAPE HAIR AMPOULE
panthenol liquid |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - PROSTEMICS Co., Ltd. (689605919) |
| Registrant - PROSTEMICS Co., Ltd. (689605919) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Prostemics Co., Ltd. Factory | 695687674 | manufacture(62041-260) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
