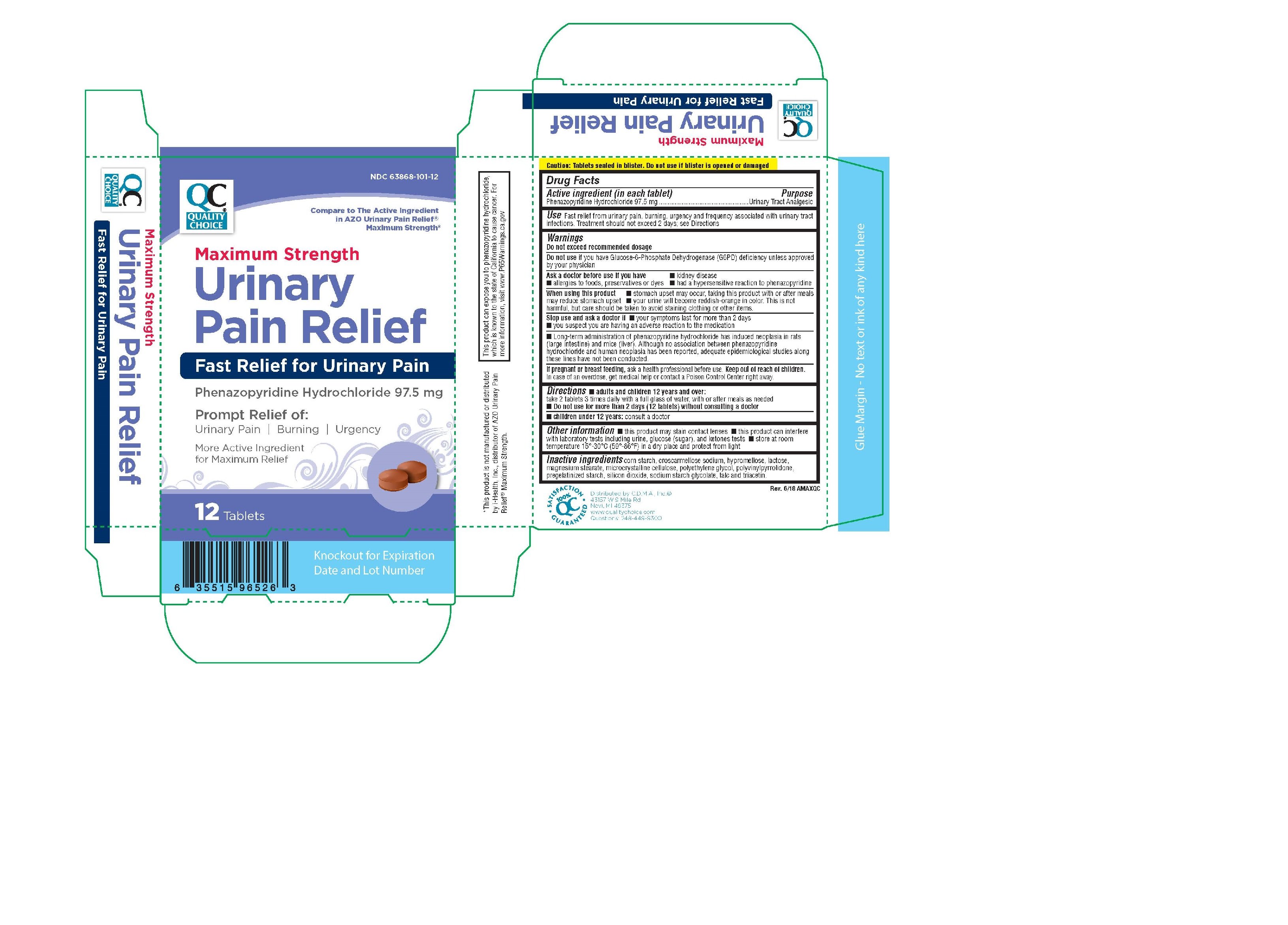
Quality Choice Maximum Strength Urinary Pain Relief
Dosage form: tablet
Ingredients: PHENAZOPYRIDINE HYDROCHLORIDE 97.5mg
Labeler: Chain Drug Marketing Association
NDC code: 63868-101
Medically reviewed by Drugs.com. Last updated on Jan 20, 2025.
Phenazopyridine Hydrochloride 97.5 mg .
Urinary Analgesic
Do not exceed recommended dosage
■ kidney disease
■ allergies to food, preservatives or dyes
■ had a hypersensitive reaction to phenazopyridine
■ stomach upset may occur, taking this product with or after meals may
reduce stomach upset
■ your urine will become reddish-orange in color. This is not harmful, but
care should be taken to avoid staining clothing or other items.
■ your symptoms last for more than 2 days
■ you suspect you are having an adverse reaction to the medication
Ask a health professional before use.
In case of an overdose, get medical help or contact a Poison Control Center right away.
Fast relief from urinary pain, burning, urgency and frequency associated with urinary tract
infections.
Lactose, magnesium silicate, magnesium stearate, microcrystalline cellulose, pharmaceutical glaze, and sodium starch glycolate.
■ adults and children 12 years and over:
take 2 tablets 3 times daily with a full glass of water, with or after meals as needed
■ children under 12 years: consult a doctor
■ Do not use for more than 2 days (12 tablets) without consulting a doctor
| QUALITY CHOICE MAXIMUM STRENGTH URINARY PAIN RELIEF
phenazopyridine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Chain Drug Marketing Association (011920774) |
| Registrant - Reese Pharmaceutical Co (004172052) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Reese Pharmaceutical Co | 004172052 | relabel(63868-101), repack(63868-101) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
