ANIOSGEL 85 NPC
Dosage form: gel
Ingredients: ALCOHOL 377.5mL in 500mL
Labeler: Laboratoires Anios
NDC code: 62169-203
Medically reviewed by Drugs.com. Last updated on Jan 24, 2025.
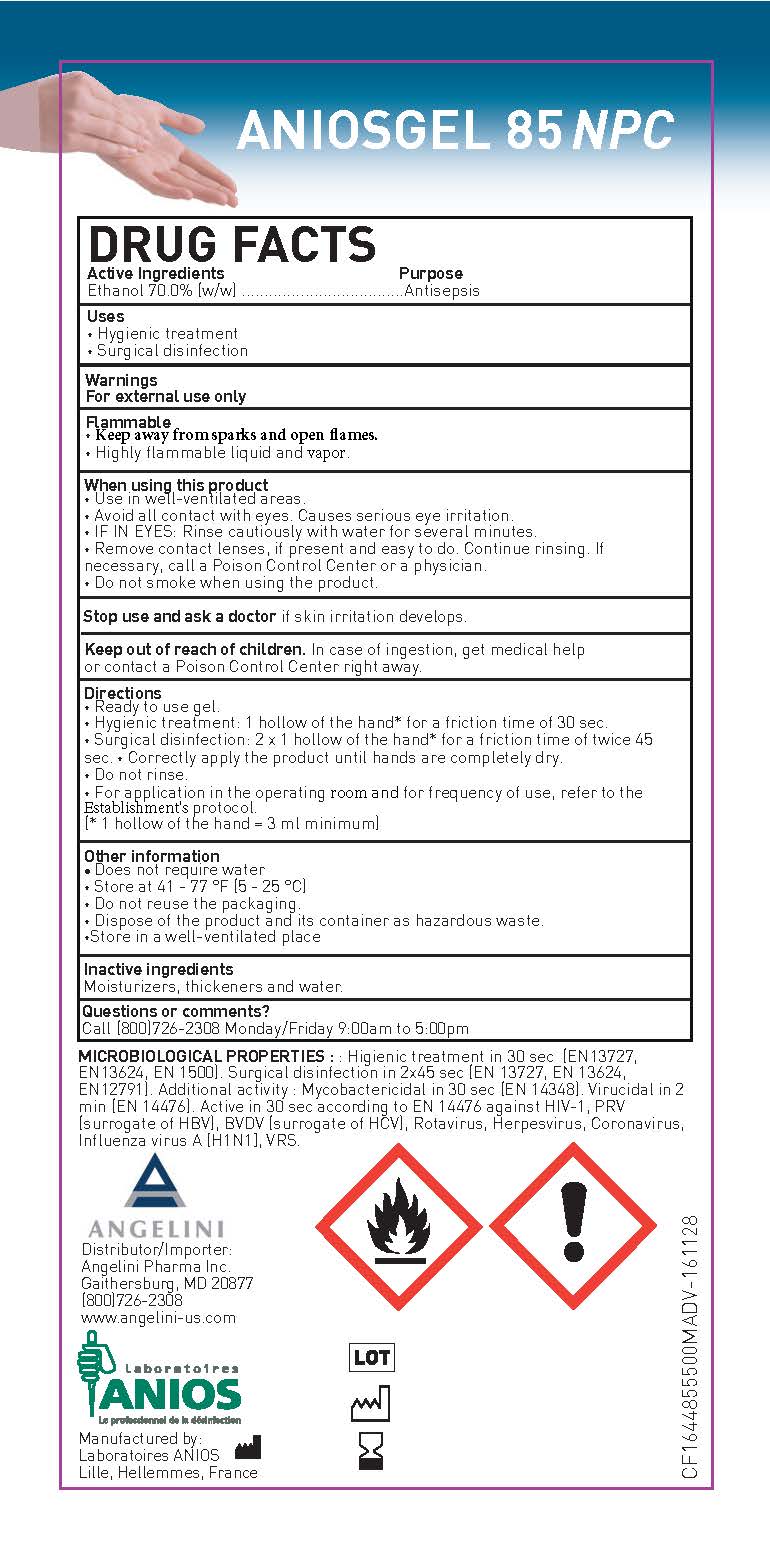
Ethanol 70.0% (w/w)
Purpose
Antisepsis
Uses
• Hygienic treatment
• Surgical disinfection
Warnings
For external use only
Flammable
• Keep away from sparks and open flames.
• Highly flammable liquid and vapor.
When using this product
• Use in well-ventilated areas.
• Avoid all contact with eyes. Causes serious eye irritation.
• IF IN EYES: Rinse cautiously with water for several minutes.
• Remove contact lenses, if present and easy to do. Continue rinsing. If
necessary, call a Poison Control Center or a physician.
• Do not smoke when using the product.
Stop use and ask a doctor if skin irritation develops.
Keep out of reach of children.
In case of ingestion, get medical help or contact a Poison Control Center right away.
Directions
• Ready to use gel.
• Hygienic treatment: 1 hollow of the hand* for a friction time of 30 sec.
• Surgical disinfection: 2 x 1 hollow of the hand* for a friction time of twice 45
sec. • Correctly apply the product until hands are completely dry.
• Do not rinse.
• For application in the operating room and for frequency of use, refer to the
Establishment's protocol.
(* 1 hollow of the hand = 3 ml minimum)
Other information
• Does not require water
• Store at 41 - 77 °F (5 - 25 °C)
• Do not reuse the packaging.
• Dispose of the product and its container as hazardous waste.
•Store in a well-ventilated place
Inactive ingredients
Moisturizers, thickeners and water.
Questions or comments?
Call (800)726-2308 Monday/Friday 9:00am to 5:00pm
DRUG FACTS
Main Display
| ANIOSGEL 85
NPC
ethanol gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Laboratoires Anios (268309216) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Laboratoires Anios | 268309216 | manufacture(62169-203), label(62169-203) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
