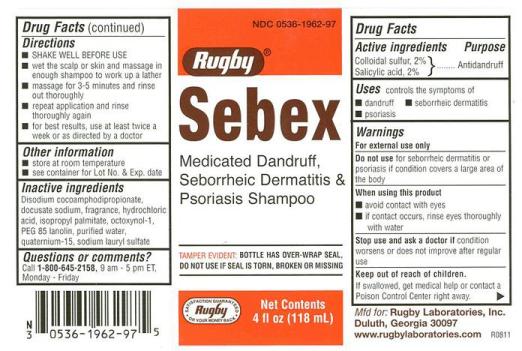
Sebex
Dosage form: shampoo
Ingredients: salicylic acid 20mg in 1000mL, sulfur 20mg in 1000mL
Labeler: Rugby Laboratories
NDC code: 0536-1962
Medically reviewed by Drugs.com. Last updated on Dec 10, 2024.
Colloidal sulfur, 2%
Salicylic acid, 2%
Antidandruff
- Controls the symptoms of:
-Dandruff
-Seborrheic dermatitis
-Psoriasis
For external use only
For external use only
Do not use for seborrheic dermatitis or psoriasis if condition covers a large area of the body
- avoid contact with eyes
- if contact occurs, rise eyes thoroughly with water
condition worsens or does not improve after regular use
If swallowed, get meidcal help or contact a Poison Control Center right away.
- SHAKE WELL BEFORE USE
- wet the scalp or skin and massage in enough shampoo to work up a lather
- massage for 3-5 minutes and rinse out thoroughly
- repeat application and rinse thoroughly again
- for best results, use at least twice a week or as directed by a doctor
- store at room temperature
- see container for Lot Number and Expiration Date
Disodium cocoamphodipropionate, docusate sodium, fragrance, hydrochloric acid, isopropyl palmitate, octoxynol-1, PEG 85 lanolin, purified water, quaternium-15, sodium lauryl sulfate
Questions or comments?
Call 1-800-645-2158
SEBEX
Medicated Dandruff, Seborrheic Dermatitis &
Psoriasis Shampoo
| SEBEX
salicylic acid, colloidal sulfur shampoo |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Rugby Laboratories (079246066) |
| Registrant - Garcoa, Inc. (036464697) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sigan Industries, Inc. | 255106239 | MANUFACTURE(0536-1962) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
