Cough Syrup DM
Dosage form: syrup
Ingredients: DEXTROMETHORPHAN HYDROBROMIDE 10mg in 5mL, GUAIFENESIN 100mg in 5mL
Labeler: Zee Medical Inc
NDC code: 35418-119
Medically reviewed by Drugs.com. Last updated on Sep 23, 2024.
Active Ingredient (in each 5 ml) Guaifenesin 100 mg, Dextromethorphan HBr 10 mg
Purpose-Cough Suppressant, Expectorant
Uses ■ temporarily relieves cough due to minor throat and
bronchial irritation that occur with a cold or inhaled irritants
■ helps loosen phlegm (mucus) and thin bronchial secretions to
make coughs more productive
Directions
- shake well before using
- adults and children over 12 years: take
2 teaspoons (one packet) every 4 hours
- do not exceed 12 teaspoons (6 packets) in 24 hours
- children under 12 years: do not use
Warnings
Do not use if you are now taking a prescription monoamine
oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric,
or emotional conditions, or Parkinson's disease), or for 2 weeks
after stopping the MAOI drug. If you do not know if your
prescription drug contains an MAOI, ask a doctor or pharmacist
before taking this product.
Stop use and ask a doctor if cough lasts more than 7 days,
comes back, or is accompanied by fever, rash, or persistent
headache. These could be signs of a serious condition.➧
Ask a doctor before use if you have
■ cough that occurs with too much phlegm (mucus)
■ cough that lasts or is chronic such as occurs with smoking,
asthma, chronic bronchitis, or emphysema
If pregnant or breast-feeding baby, ask a health professional
before use
KEEP OUT OF REACH OF CHILDREN. In case of overdose,
get medical help or contact a Poison Control Center right away.
Prompt medical attention is critical for adults as well as for
children even if you do not notice any signs or symptoms.
Inactive ingredients
citric acid, FDC red 40, flavor, glycerin, methyl
paraben, propylene glycol, propyl paraben, purified
water, sodium citrate, sucralose
MM1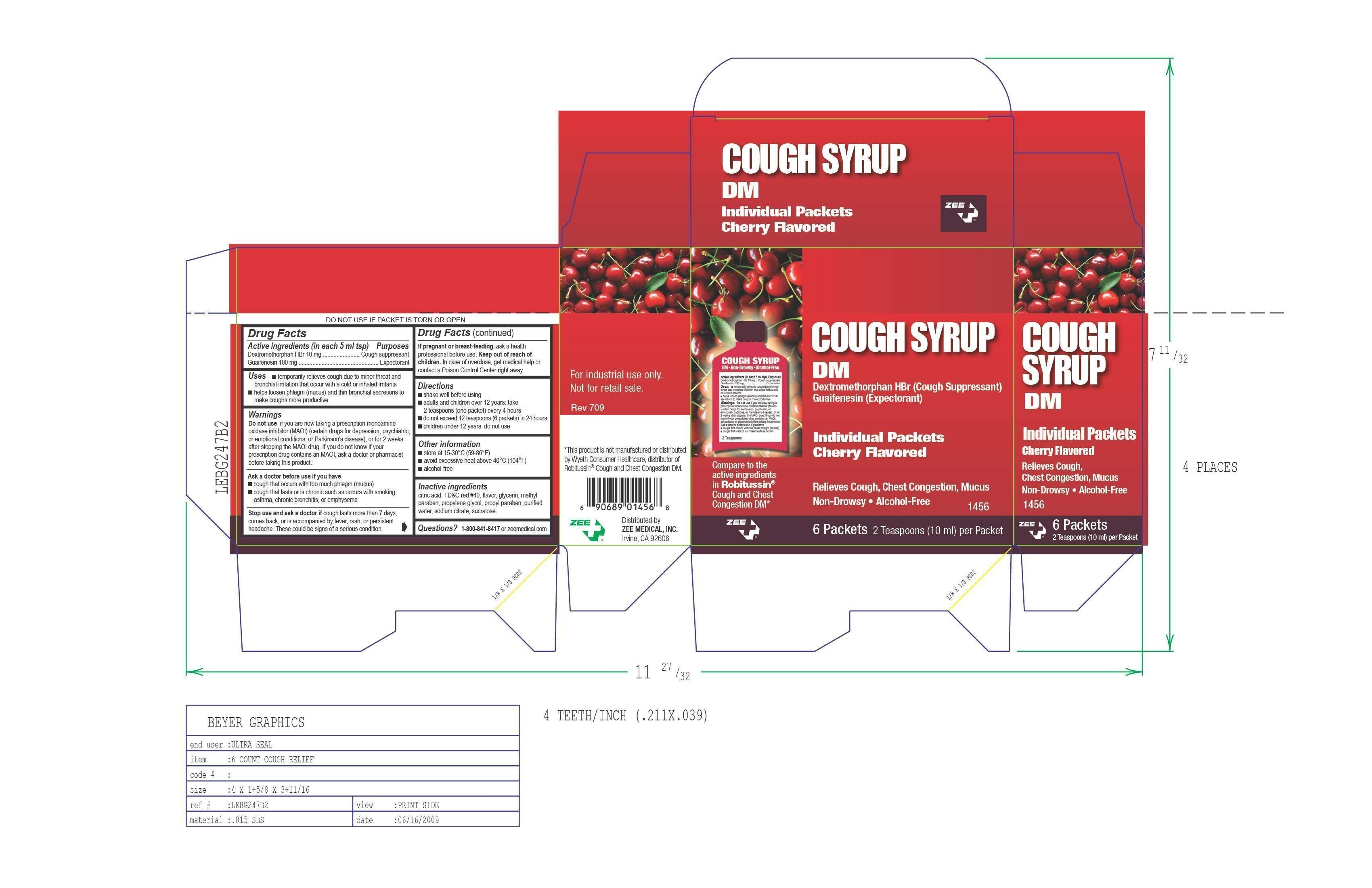 1
1
| COUGH SYRUP DM
guaifenesin, dextromethorpham hbr syrup |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Zee Medical Inc (009645623) |
| Registrant - Ultra Seal Corporation (085752004) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Ultratab Laboratories, Inc. | 151051757 | manufacture(35418-119) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Ultra Seal Corporation | 085752004 | repack(35418-119) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
