Y LAX DR
Dosage form: tablet, sugar coated
Ingredients: BISACODYL 5mg
Labeler: SPIRIT PHARMACEUTICALS,LLC
NDC code: 68210-0001
Medically reviewed by Drugs.com. Last updated on Apr 11, 2025.
Drug Facts
Bisacodyl 5 mg
Stimulant laxative
- for relief of occasional constipation(irregularity)
- this product generally produces bowel movement in 6 to 12 hours
Do not use if you cannot swallow without chewing
Ask a doctor before use if you have
- Stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that persists over a period of 2 weeks
When using this product
- do not chew or crush tablets
- do not take this product within 1 hour after taking an antacid or milk
- it may cause stomach discomfort,faintness, and cramps
Stop use and ask doctor if
- you need to use more than 1 week
- rectal bleeding or failure to have a bowel movement occur after use of a laxative.These may be signs of a serious condition
If pregana or breast-feeding, ask a health professional before use
Keep out of reach of children.In case of overdose,get medical help or contact a poison control center right away.
take with a glass of water
- Do not take more than three tablets daily
| adults and children 12 years of age and older | - take 1 to 3 tablets in a single dose once daily |
| children 6 to under 12 years of age | - take 1 tablet once daily |
| children under 6 years of age | - ask a doctor |
- store between 20° to 25°C (68° to 77° F)
- protection from excessive humidity
lactose,cornstarch,povidone (K-30),sodium startch glycolate,talc,magnesium stearate,methacrylic acid copolymer,polethylene glaycol,sodium hydroxide pellets, sucrose,acacia,gelatin,methylparaben, propylparaben, calcium sulphate dihydrate,titanium dioxide,D&C yellow #6 lake, FD & C yellow #10; pharmaceuticals glaze
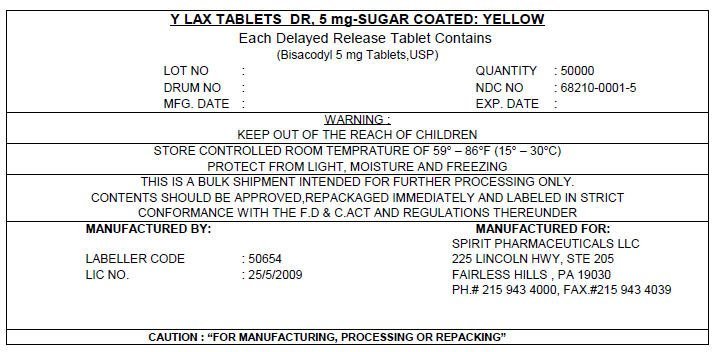
Y LAX TABLETS DR, 5 mg-SUGAR COATED: YELLOW
Each Delayed Release Tablet Contains
(Bisacodyl 5 mg Tablets,USP)
LOT NO :
DRUM NO :
MFG. DATE :
QUANTITY : 50000
NDC NO : 68210-0001-5
EXP. DATE :
WARNING :
KEEP OUT OF THE REACH OF CHILDREN
STORE CONTROLLED ROOM TEMPRATURE OF 59° – 86°F (15° – 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZING
THIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED,REPACKAGED IMMEDIATELY AND LABELED IN STRICT
CONFORMANCE WITH THE F.D & C.ACT AND REGULATIONS THEREUNDER
MANUFACTURED BY:
LABELLER CODE : 50654
LIC NO. : 25/5/2009
MANUFACTURED FOR:
SPIRIT PHARMACEUTICALS LLC
225 LINCOLN HWY, STE 205
FAIRLESS HILLS , PA 19030
PH.# 215 943 4000, FAX.#215 943 4039
CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"
| Y LAX DR
bisacodyl tablet, sugar coated |
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| Labeler - SPIRIT PHARMACEUTICALS,LLC (179621011) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| MISSION VIVACARE LIMITED | 677604252 | API MANUFACTURE, RECOVERY | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
