TCL 093 Pill: beige, round
The pill with imprint TCL 093 (Beige, Round, 0mm) has been identified as Ferrous Sulfate ER 160 mg and is used for Iron Deficiency Anemia, Anemia Due to Chronic Kidney Disease, Vitamin/Mineral Supplementation and Deficiency, and Vitamin/Mineral Supplementation during Pregnancy/Lactation. It belongs to the drug class iron products and is not a controlled substance.
Images for TCL 093
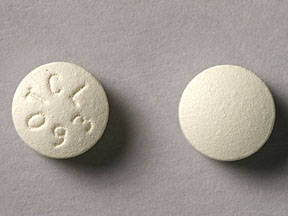
Ferrous Sulfate ER
- Imprint
- TCL 093
- Strength
- 160 mg
- Color
- Beige
- Shape
- Round
- Availability
- Rx and/or OTC
- Drug Class
- Iron products
- Pregnancy Category
- N - Not classified
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Watson Pharmaceuticals
- National Drug Code (NDC)
- 00536-3478 (Discontinued)
See also:
More about ferrous sulfate
- Check interactions
- Compare alternatives
- Reviews (48)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: iron products
Patient resources
- Ferrous Sulfate drug information
- Ferrous Sulfate Capsules and Tablets
- Ferrous Sulfate Drops
- Iron Tablets and Capsules
- Ferrous Sulfate Liquid
Other brands
FeroSul, Slow Fe, Feosol Original, Fer-In-Sol, ... +5 more
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
