IP 272 Pill: white, oval, 19mm
Generic Name: sulfamethoxazole/trimethoprim
The pill with imprint IP 272 (White, Oval, 19mm) has been identified as Sulfamethoxazole and trimethoprim DS 800 mg / 160 mg and is used for Bacterial Skin Infection, Bacterial Infection, Diverticulitis, Bronchitis, and Epiglottitis. It belongs to the drug class sulfonamides and is not a controlled substance.
Images for IP 272
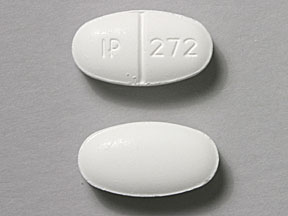
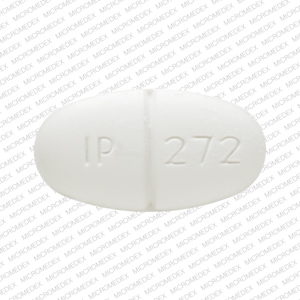



Sulfamethoxazole and trimethoprim DS
- Generic Name
- sulfamethoxazole/trimethoprim
- Imprint
- IP 272
- Strength
- 800 mg / 160 mg
- Color
- White
- Size
- 19.00 mm
- Shape
- Oval
- Availability
- Prescription only
- Drug Class
- Sulfonamides
- Pregnancy Category
- D - Positive evidence of risk
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Amneal Pharmaceuticals
- Inactive Ingredients
-
magnesium stearate,
povidone,
corn starch,
sodium starch glycolate type A potato
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00904-2725 | Major Pharmaceuticals |
| 42291-0759 | AvKare, Inc. |
| 53746-0272 | Amneal Pharmaceuticals LLC |
| 68084-0230 (Discontinued) | Amerisource Health Services |
| 33261-0148 | Aidarex Pharmacuticals, LLC (repackager) |
| 54569-0075 (Discontinued) | A-S Medication Solutions, LLC (repackager) |
| 54569-5813 (Discontinued) | A-S Medication Solutions, LLC (repackager) |
| 55289-0241 (Discontinued) | PDRX Pharmaceuticals Inc. (repackager) |
See also:
More about SMZ-TMP DS (sulfamethoxazole / trimethoprim)
- Check interactions
- Compare alternatives
- Reviews (54)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: sulfonamides
Patient resources
Other brands
Bactrim, Septra, Septra DS, Sulfatrim, ... +2 more
Professional resources
Other brands
Bactrim, Septra, Co-trimoxazole, Sulfatrim
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
