G 531 Pill: white, round, 9mm
The pill with imprint G 531 (White, Round, 9mm) has been identified as Bendroflumethiazide and Nadolol 5 mg / 40 mg and is used for High Blood Pressure. It belongs to the drug class beta blockers with thiazides and is not a controlled substance.
Images for G 531
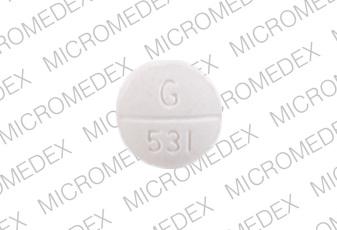

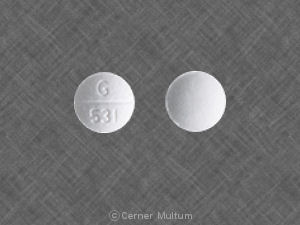

Bendroflumethiazide and Nadolol
- Imprint
- G 531
- Strength
- 5 mg / 40 mg
- Color
- White
- Size
- 9.00 mm
- Shape
- Round
- Availability
- Prescription only
- Drug Class
- Beta blockers with thiazides
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Amneal Pharmaceuticals LLC
- National Drug Code (NDC)
- 00115-5311 (Discontinued)
- Inactive Ingredients
-
croscarmellose sodium,
hypromelloses,
lactose monohydrate,
magnesium stearate,
mannitol,
microcrystalline cellulose,
corn starch,
magnesium silicate
Note: Inactive ingredients may vary.
Related images for "G 531"
More about bendroflumethiazide / nadolol
- Check interactions
- Compare alternatives
- Reviews (1)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: beta blockers with thiazides
- En español
Patient resources
- Bendroflumethiazide and nadolol drug information
- Nadolol and bendroflumethiazide (Advanced Reading)
- Nadolol and Bendroflumethiazide
Other brands
Corzide, Corzide 40/5, Corzide 80/5
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.