BRX 0.5 Pill: orange, round, 6mm
Generic Name: brexpiprazole
The pill with imprint BRX 0.5 (Orange, Round, 6mm) has been identified as Rexulti 0.5 mg and is used for Major Depressive Disorder, Agitation Associated with Dementia Due to Alzheimer’s Disease, Schizophrenia, and Depression. It belongs to the drug class atypical antipsychotics and is not a controlled substance.
Images for BRX 0.5
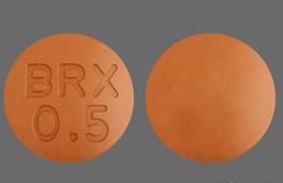
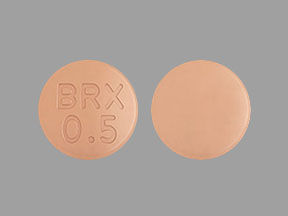
Rexulti
- Generic Name
- brexpiprazole
- Imprint
- BRX 0.5
- Strength
- 0.5 mg
- Color
- Orange
- Size
- 6.00 mm
- Shape
- Round
- Availability
- Prescription only
- Drug Class
- Atypical antipsychotics
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Otsuka Pharmaceutical Co., Ltd. and Lundbeck
- National Drug Code (NDC)
- 59148-0036
- Inactive Ingredients
-
lactose monohydrate,
corn starch,
microcrystalline cellulose,
magnesium stearate,
magnesium silicate,
titanium dioxide,
ferric oxide red,
ferric oxide yellow
Note: Inactive ingredients may vary.
Related images for "BRX 0.5"
More about Rexulti (brexpiprazole)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (502)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Generic availability
- Support group
- FDA approval history
- Drug class: atypical antipsychotics
- Breastfeeding
- En español
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.