Generic Suprenza Availability
Last updated on Jul 9, 2025.
Suprenza is a brand name of , approved by the FDA in the following formulation(s):
SUPRENZA (phentermine hydrochloride - tablet, orally disintegrating;oral)
-
Manufacturer: CITIUS PHARMS
Approval date: June 13, 2011
Strength(s): 15MG (discontinued), 30MG (discontinued) -
Manufacturer: CITIUS PHARMS
Approval date: March 27, 2012
Strength(s): 37.5MG (discontinued)
All of the above formulations have been discontinued.
Note: Fraudulent online pharmacies may attempt to sell an illegal generic version of Suprenza. These medications may be counterfeit and potentially unsafe. If you purchase medications online, be sure you are buying from a reputable and valid online pharmacy. Ask your health care provider for advice if you are unsure about the online purchase of any medication.
See also: Generic Drug FAQ.
Related patents
Patents are granted by the U.S. Patent and Trademark Office at any time during a drug's development and may include a wide range of claims.
-
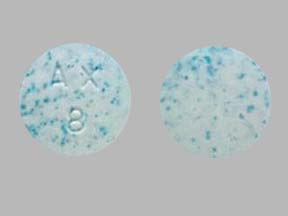
Orally disintegrating tablets with speckled appearance
Patent 8,440,170
Issued: May 14, 2013
Inventor(s): Stroppolo Federico & Ardalan Shahbaz
Assignee(s): Alpex Pharma SAOrally disintegrating tablets containing colored granules of a water-soluble sugar which give them a speckled appearance are described. The orally disintegrating tablets with speckled appearance are readily and easy identifiable by physicians, nurses and patients.
Patent expiration dates:
- March 14, 2029✓
- March 14, 2029
More about Suprenza (phentermine)
- Suprenza consumer information
- Check interactions
- Compare alternatives
- Reviews (1)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: anorexiants
- Breastfeeding
Related treatment guides
Related/similar drugs
Ozempic
Learn about Ozempic (semaglutide) for type 2 diabetes treatment, weight management, cardiovascular ...
Mounjaro
Mounjaro is used for type 2 diabetes to help lower blood sugar levels. Mounjaro has also been shown ...
Wegovy
Wegovy (semaglutide) an FDA-approved weekly injection for weight loss and to reduce heart risks ...
Rybelsus
Rybelsus tablets are used to improve blood sugar control in adults with type 2 diabetes, and may ...
Zepbound
Zepbound (tirzepatide) is an FDA-approved weekly injection for weight loss and obstructive sleep ...
Victoza
Victoza helps control blood sugar levels and reduce the risk of serious heart problems in people ...
Saxenda
Saxenda (liraglutide) injection is used for weight loss in obese or overweight patients. Includes ...
Alli
alli blocks the absorption of some of the fat that you eat and is used to treat obesity. Learn ...
Amphetamine
Amphetamine is a stimulant and is used to trat narcolepsy and attention deficit disorder. Includes ...
Tirzepatide
Tirzepatide is a once-weekly injection used for weight loss, sleep apnea, and type 2 diabetes ...
Glossary
| Term | Definition |
|---|---|
| Drug Patent | A drug patent is assigned by the U.S. Patent and Trademark Office and assigns exclusive legal right to the patent holder to protect the proprietary chemical formulation. The patent assigns exclusive legal right to the inventor or patent holder, and may include entities such as the drug brand name, trademark, product dosage form, ingredient formulation, or manufacturing process A patent usually expires 20 years from the date of filing, but can be variable based on many factors, including development of new formulations of the original chemical, and patent infringement litigation. |
| Drug Exclusivity | Exclusivity is the sole marketing rights granted by the FDA to a manufacturer upon the approval of a drug and may run simultaneously with a patent. Exclusivity periods can run from 180 days to seven years depending upon the circumstance of the exclusivity grant. |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.