RX657 RX657 Pill - dark & light green capsule/oblong, 22mm
Pill with imprint RX657 RX657 is Dark & Light Green, Capsule/Oblong and has been identified as Cephalexin 500 mg. It is supplied by Ranbaxy Pharmaceuticals Inc.
Cephalexin is used in the treatment of Bladder Infection; Bacterial Infection; Bacterial Endocarditis Prevention; Bone infection; Kidney Infections and belongs to the drug class first generation cephalosporins. There is no proven risk in humans during pregnancy. Cephalexin 500 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for RX657 RX657
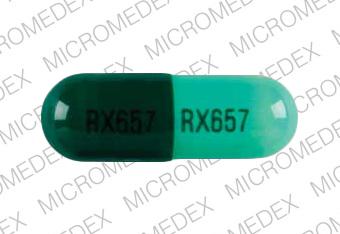
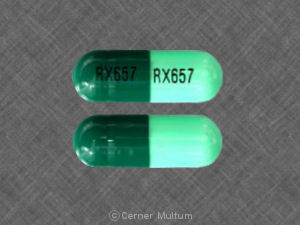
Cephalexin
- Imprint
- RX657 RX657
- Strength
- 500 mg
- Color
- Dark & Light Green
- Size
- 22.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- First generation cephalosporins
- Pregnancy Category
- B - No proven risk in humans
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Ranbaxy Pharmaceuticals Inc.
- National Drug Code (NDC)
- 63304-0657 (Discontinued)
See also:
Keflex
Keflex (cephalexin) is used to treat infections caused by bacteria, including respiratory ...
Macrobid
Macrobid (nitrofurantoin) is an antibiotic used to treat urinary tract infections. Includes side ...
Botox
Botox is used cosmetically to reduce facial lines and wrinkles and for medical purposes for ...
Cipro
Cipro (ciprofloxacin) is a fluoroquinolone antibiotic used to treat bacterial infections. Learn ...
Levaquin
Levaquin (levofloxacin) is used to treat bronchitis, pneumonia, chlamydia, gonorrhea and skin ...
Tetracycline
Tetracycline is an antibiotic used to treat bacterial infections such as urinary tract infections ...
Cefuroxime
Cefuroxime is used for bacterial infection, bladder infection, bone infection, bronchitis ...
Cefixime
Cefixime is an antibiotic that may be used to treat many different types of infections caused by ...
Nitrofurantoin
Nitrofurantoin is an antibiotic used to treat urinary tract infections. Learn about side effects ...
Levofloxacin
Levofloxacin is a fluoroquinolone antibiotic used to treat serious bacterial infections and prevent ...
More about cephalexin
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (510)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: first generation cephalosporins
- Breastfeeding
- En español
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
