Vizz: Package Insert / Prescribing Info
Package insert / product label
Generic name: aceclidine
Dosage form: ophthalmic solution
Drug class: Miscellaneous ophthalmic agents
Medically reviewed by Drugs.com. Last updated on Aug 19, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
VIZZ (aceclidine ophthalmic solution) 1.44%, for topical ophthalmic use
Initial U.S. Approval: 2025
Indications and Usage for Vizz
VIZZ is a cholinergic agonist indicated for the treatment of presbyopia in adults ( 1).
Vizz Dosage and Administration
Instill one drop in each eye, wait 2 minutes and instill a second drop in each eye once daily ( 2).
Dosage Forms and Strengths
Ophthalmic solution: aceclidine 1.44% in a single-dose vial ( 3).
Contraindications
None. (4)
Warnings and Precautions
- Blurred Vision:Patients may experience temporary dim or dark vision after instillation. Do not drive or operate machinery if vision is not clear (e.g., blurred vision). Exercise caution in night driving and other hazardous activities in poor illumination ( 5.1).
- Risk of Retinal Tear/Detachment:Rare cases of retinal tears and detachments have been reported with miotics. Examination of the retina is advised in all patients prior to initiation of therapy. Patients should be advised to seek immediate medical care with sudden onset of flashing lights, floaters, or vision loss ( 5.2).
- Iritis: Caution is advised in patients with a history of iritis ( 5.3).
Adverse Reactions/Side Effects
The most common adverse reactions were instillation site irritation (20%), dim vision (16%), and headache (13%) ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact LENZ Therapeutics, Inc. at 1-888-711-LENZ or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2025
Full Prescribing Information
2. Vizz Dosage and Administration
2.1 Recommended Dosage
Instill one drop in each eye, wait 2 minutes and instill a second drop in each eye once daily from the same single-dose vial.
2.2 Administration Instructions
Contact lenses should be removed prior to the instillation of VIZZ, and may be reinserted 10 minutes after instillation.
If more than one topical ophthalmic product is being used, the products should be administered at least 5 minutes apart.
To open vial, twist off top as shown.
Discard the opened single-dose vial after use.
3. Dosage Forms and Strengths
VIZZ is a clear to opalescent and colorless to slightly yellow ophthalmic solution containing 1.44% of aceclidine in a single-dose vial.
5. Warnings and Precautions
5.1 Blurred Vision
Miotics may cause accommodative spasm. Do not drive or operate machinery if vision is not clear (e.g., blurred vision).
Patients may experience temporary dim or dark vision. Exercise caution in night driving and other hazardous activities in poor illumination.
5.2 Risk of Retinal Tear/Detachment
Rare cases of retinal tear and detachment have been reported with miotics when used in susceptible individuals and those with pre-existing retinal disease. Examination of the retina is advised in all patients prior to the initiation of treatment with VIZZ. Patients should be advised to seek immediate care with sudden onset of flashing lights, floaters, or vision loss.
5.3 Iritis
Sequelae of ocular inflammation, i.e., adhesions (synechiae) between the iris and the lens, may be exacerbated with miotic use in patients with a known history of iritis.
5.4 Hypersensitivity
VIZZ is not recommended for use in patients with a known hypersensitivity to aceclidine or any other ingredient in VIZZ.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
VIZZ dosed once daily was evaluated for safety and efficacy in 466 participants with presbyopia in 2 randomized, double-masked, controlled phase 3 studies for 42 days (CLARITY-1, NCT05656027 and CLARITY-2, NCT05728944). VIZZ dosed once daily was also evaluated for long term safety in 217 participants with presbyopia in a separate randomized, double-masked, controlled phase 3 study (CLARITY-3, NCT05753189) for a 6-month duration.
The most common reported adverse reactions of participants were instillation site irritation (20%), dim vision (16%), and headache (13%). Adverse reactions reported in > 5% of participants were conjunctival hyperemia (8%) and ocular hyperemia (7%). The majority of adverse reactions were mild, transient, and self-resolving.
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no adequate and well controlled studies of VIZZ administration in pregnant women to inform a drug associated risk. In animal reproduction studies, oral administration of aceclidine to pregnant rats and rabbits throughout organogenesis and lactation did not produce adverse maternal, fetal or neonatal effects at clinically relevant doses.
Data
Animal Data
In embryofetal development studies, oral administration of aceclidine to pregnant rats and rabbits throughout organogenesis produced no maternal toxicity, skeletal anomalies, nor reduction in fetal body weight at 1.5 mg/kg/day (approximately 110-fold and 70-fold the human plasma exposure to the metabolite, 3-quinuclidinol, in rats and rabbits, respectively, at the MRHOD, assuming administration of 2 drops/eye/day).
In a pre-/postnatal development study in rats, oral administration of aceclidine during organogenesis through lactation produced no adverse maternal, fetal, or neonatal effects at doses up to 1.5 mg/kg/day (approximately 110-fold higher than the MHOD based on body surface area, assuming administration of 2 drops/eye/day).
8.2 Lactation
Risk Summary
There is no information regarding the presence of VIZZ or its metabolite in human or animal milk, the effects on breastfed infants or the effects on milk production to inform the risk of VIZZ to an infant during lactation.
Systemic levels of aceclidine and its metabolites following topical ocular administration are low [see Clinical Pharmacology (12.3)] , and it is not known whether measurable levels of aceclidine or its metabolites would be present in human milk following topical ocular administration.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for VIZZ and any potential adverse effects on the breastfed child from VIZZ or from the underlying maternal condition.
10. Overdosage
Systemic toxicity following topical ocular administration of miotics is rare, but occasionally patients who are sensitive may develop increased salivation, sweating, gastrointestinal overactivity, and slowing of the pulse. Accidental ingestion can produce sweating, salivation, nausea, tremors, slowing of the pulse, and a decrease in blood pressure. In moderate overdosage, spontaneous recovery is to be expected and is aided by intravenous fluids to compensate for dehydration.
11. Vizz Description
VIZZ (aceclidine ophthalmic solution) 1.44% is a cholinergic muscarinic receptor agonist supplied as a sterile, clear to opalescent, colorless to slightly yellow, and viscous ophthalmic solution containing 1.75% of aceclidine hydrochloride (equivalent to 1.44% aceclidine).
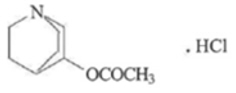
The chemical names for aceclidine hydrochloride are: 1) 3-Acetoxyquinuclidine Hydrochloride; 2) 3-Quinuclidinyl Acetate Hydrochloride. Its molecular weight is 205.68 g/mol and its molecular formula is C 9H 15NO 2∙ HCl.
Each mL of VIZZ contains aceclidine hydrochloride 1.75% (17.82 mg) as the active ingredient. Inactive ingredients in the ophthalmic solution are: polysorbate 80, mannitol, hypromellose, edetate disodium dihydrate, sodium citrate dihydrate, water for injection, and may also include hydrochloric acid and/or sodium hydroxide for pH adjustment to between 4.5 and 5.5, if necessary. VIZZ does not contain anti-microbial preservatives.
12. Vizz - Clinical Pharmacology
12.1 Mechanism of Action
Aceclidine is a cholinergic muscarinic agonist that stimulates muscarinic receptors located on smooth muscles. VIZZ is a predominantly pupil selective miotic that interacts with the iris with minimal ciliary muscle stimulation. VIZZ causes contraction of the iris sphincter muscle, resulting in a pinhole effect that extends depth of focus to improve vision.
12.3 Pharmacokinetics
Aceclidine undergoes hydrolysis in the eye to acetate and 3-quinuclidinol (3-Q) with one mole of aceclidine hydrolyzed to one mole of 3-Q. Pharmacokinetic studies are performed on the analysis of 3-Q, which is a metabolite formed from the hydrolysis of aceclidine.
Systemic exposure of aceclidine hydrochloride was evaluated in 16 subjects with presbyopia following once daily VIZZ administration (one drop of VIZZ in each eye followed by a second drop in each eye two minutes later) for 8 days. The mean C maxand AUC 0-tvalues for 3-Q after Day 8 dosing were 2.114 ng/mL and 4.899 hr*ng/mL, respectively.
There was little to no accumulation of 3-Q after repeat once daily dosing of VIZZ. After 8 days, the mean (SD) RAUC 0-tand RC maxvalues were 1.189 (0.770) and 0.996 (0.314), respectively. T 1/2could not be estimated at any timepoint due to the limited amount of quantifiable 3-Q concentrations after ocular dosing.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long term studies in animals have not been performed to evaluate carcinogenic potential.
Mutagenesis
Aceclidine did not show any potential to cause genetic toxicity in a series of studies that included: 1) bacterial assays (Salmonella and E. coli) for reverse gene mutations; 2) an in vitro chromosome aberration assay in cultured human peripheral blood lymphocytes; and 3) an in vivo chromosome aberration assay (micronucleus test) in mice.
14. Clinical Studies
The efficacy of VIZZ for the treatment of presbyopia was demonstrated in two, randomized, double-masked, controlled studies, CLARITY-1 and CLARITY-2. A total of 466 participants aged 45 to 75 years old with presbyopia were randomized. Participants had a refractive range from -4.00 to +1.00 D sphere, with astigmatism up to 2.00 D, and a spherical equivalent no more myopic than -4.00 D. Both studies included participants who were post-refractive surgery and/or pseudophakic.
Participants were instructed to instill 2 drops of VIZZ (or control) in each eye once daily, one drop in each eye followed by a second drop in each eye two minutes later. Participants were treated for 42 days. Ophthalmic efficacy assessments were conducted on Day 1, Day 15, and Day 28 of the study at various timepoints through 10 hours post dose.
In each study, the proportion of participants gaining 3 lines or more in high contrast, distance corrected, near visual acuity (DCNVA) at 40 cm, without loss of 1 line or more (≥5 letters) of distance corrected, distance visual acuity (DCDVA) at 4 meters was statistically significantly greater in the VIZZ group compared to the control group at Day 1, Hour 3.
| CLARITY-1 | CLARITY-2 | |||||
|---|---|---|---|---|---|---|
| VIZZ
N=157 | Brimonidine
N=156 | p-value | VIZZ
N=77 | Vehicle
N=76 | p-value | |
| Proportion of participants gaining 3-lines or more in DCNVA at 40cm, without losing 1 line or more (≥5 letters) of DCDVA at 4m at Day 1, at 3 hours | 65% | 12% | p<0.01 | 71% | 8% | p<0.01 |
Figure 1 and Figure 2 demonstrate the onset of the VIZZ effect on presbyopia, from 30 minutes post dose and lasting 10 hours.
Figure 1 Percentage of Participants Achieving 3 Lines or More Improvement in High Contrast, Monocular Near Vision (DCNVA at 40 cm) and No Loss of 1 or More Lines (DCDVA at 4 m) on Day 1 at All Measured Time Points (CLARITY-2, FAS population with Observed data)
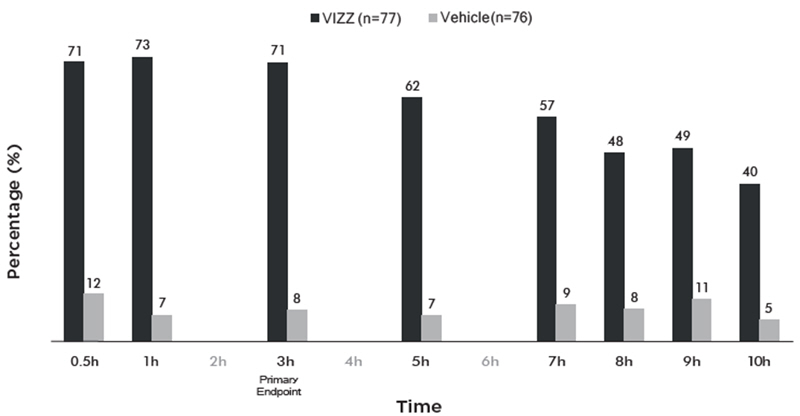
Figure 2 Percentage of Participants Achieving 3 Lines or More Improvement in High Contrast, Monocular Near Vision (DCNVA at 40 cm) and No Loss of 1 or More Lines (DCDVA at 4 m) on Day 1 at All Measured Time Points (CLARITY-1, FAS population with Observed data)
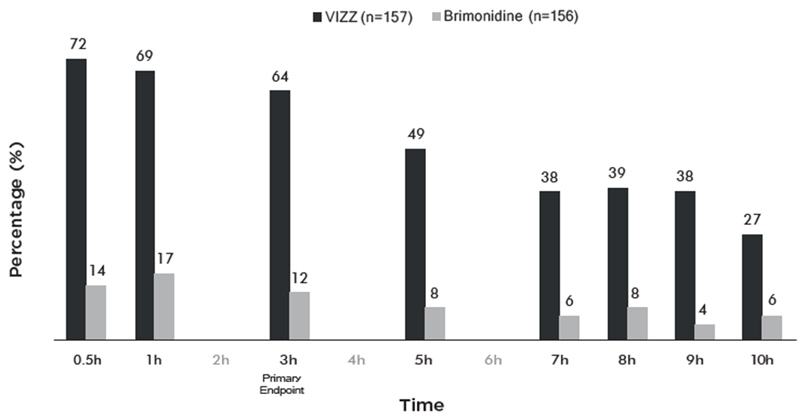
16. How is Vizz supplied
16.1 How Supplied
VIZZ (aceclidine ophthalmic solution) 1.44% is supplied as a sterile, clear to opalescent, colorless to slightly yellow, and viscous ophthalmic solution in configurations of 5 single-dose vials of 0.4 mL each. VIZZ does not include antimicrobial preservatives.
Each single-dose vial is comprised of transparent low-density polyethylene (LDPE).
5 single-dose vials are packaged in a foil pouch.
16.2 Storage and Handling
Store refrigerated at 36°F to 46°F (2°C to 8°C). Do not freeze.
When stored in refrigerated conditions, VIZZ can be used until the expiration date.
Once pouch or vial(s) are removed from refrigeration, VIZZ may be stored at room temperature [up to 77°F (25°C)] but must be used within 30 days.
Discard the opened single-dose vial after use.
During shipment, VIZZ may be maintained at temperatures up to 104°F (40°C) for a period not exceeding 8 days.
17. Patient Counseling Information
Night Driving
Advise patients that they may experience temporary dim or dark vision. Advise patients to exercise caution with night driving and when hazardous activities are undertaken in poor illumination [see Warnings and Precautions (5.1)].
Blurred Vision
Temporary problems when changing focus between near and distant objects may occur. Advise patients not to drive or use machinery if vision is not clear (e.g., blurred vision) [see Warnings and Precautions (5.1)] .
When to Seek Physician Advice
Advise patients to seek immediate medical care with sudden onset of flashing lights, floaters, or vision loss [see Warnings and Precautions (5.2)].
Contact Lens Wear
Advise patients to remove contact lenses prior to the instillation of VIZZ. Wait 10 minutes after instillation before reinserting their contact lenses [see Warnings and Precautions (5.5)].
Potential for Eye Injury or Contamination
Advise patients to avoid touching the tip of the single-dose vial to the eye or to any other surface in order to prevent eye injury or contamination [see Warnings and Precautions (5.6)]. Advise patients to discard the opened single-dose vial and any remaining contents after use.
Concomitant Topical Ocular Therapy
Advise patients if more than one topical ophthalmic medication is being used, the medicines should be administered at least 5 minutes apart [see Dosage and Administration (2)] .
| VIZZ
aceclidine solution/ drops |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - LENZ Therapeutics, Inc. (118682746) |
| Registrant - LENZ Therapeutics Operations, Inc. (070662821) |
More about Vizz (aceclidine ophthalmic)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: miscellaneous ophthalmic agents
- En español