Radiogenix System: Package Insert / Prescribing Info
Package insert / product label
Generic name: technetium tc 99m generator
Dosage form: injection
Drug class: Diagnostic radiopharmaceuticals
Medically reviewed by Drugs.com. Last updated on Jun 23, 2025.
On This Page
Indications and Usage for Radiogenix System
The RadioGenix System is a technetium Tc-99m generator used to produce sterile, non-pyrogenic Sodium Pertechnetate Tc 99m Injection. Sodium Pertechnetate Tc 99m Injection is indicated for use in the preparation of FDA-approved diagnostic radiopharmaceuticals.
Sodium Pertechnetate Tc 99m Injection is also indicated in:
Adults for:
- Thyroid Imaging
- Salivary Gland Imaging
- Urinary Bladder Imaging (direct isotopic cystography) for detection of vesicoureteral reflux
- Nasolacrimal Drainage System Imaging (dacryoscintigraphy)
Pediatric Patients for:
- Thyroid Imaging
- Urinary Bladder Imaging (direct isotopic cystography) for the detection of vesicoureteral reflux.
Radiogenix System Dosage and Administration
2.1 Radiation Safety Drug Handling
- The potassium molybdate Mo-99 source solution and Sodium Pertechnetate Tc 99m Injection are radioactive and should be handled with appropriate safety measures to minimize radiation exposure to patients and healthcare providers. Use waterproof gloves and effective shielding, including syringe shields, throughout the entire preparation and handling for the RadioGenix System and technetium Tc-99m injection [see Warnings and Precautions (5.1)].
2.2 Important Administration Instructions
- Use aseptic technique in eluting generator and in all drug preparation and handling.
- Inspect the Sodium Pertechnetate Tc 99m Injection for particulate matter and discoloration prior to administration. Do not administer Sodium Pertechnetate Tc 99m Injection if there is any evidence of particulate matter and discoloration.
- Measure patient dose with a suitable radioactivity calibration system immediately prior to administration.
- Instruct patients to hydrate after intravenous or intravesicular administration. Encourage the patient to void as soon as the imaging study is completed and frequently for the next 12 hours to minimize the radiation absorbed dose to the bladder.
- Instruct patients to blow their nose and/or wash their eyes with sterile distilled water or an isotonic sodium chloride solution after ophthalmic administration to minimize the radiation absorbed dose.
2.3 Recommended Dose for Adults
The recommended doses for adult patients are shown in Table 1.
|
Table 1 Recommended Dose of Sodium Pertechnetate for Adult Patients |
|||
|
Indication |
Megabecquerels (MBq) |
Millicuries (mCi) |
Administration Technique |
|
Vesicoureteral imaging: |
18.5 to 37 |
0.5 to 1 |
|
|
Thyroid gland imaging: |
37 to 370 |
1 to 10 |
Intravenous |
|
Salivary gland imaging: |
37 to 185 |
1 to 5 |
Intravenous |
|
Nasolacrimal drainage system imaging: |
3.7 (maximum) |
0.1 (maximum) |
Ophthalmic instillation with micropipette or similar method |
2.4 Recommended Dose for Pediatric Patients
The recommended doses for pediatric patients are shown in Table 2 [see Use in Specific Populations (8.4)].
|
Table 2 Recommended Dose of Sodium Pertechnetate for Pediatric Patients |
|||
|
Indication |
Administration Technique |
||
|
Vesicoureteral imaging: |
18.5 MBq to 37 MBq |
0.5 mCi to 1 mCi |
Intravesicular via urethral catheter |
|
Thyroid gland imaging: |
2.2 MBq/kg to 2.96 MBq/kg (370 MBq maximum) |
0.06 mCi/kg to 0.08 mCi/kg (10 mCi maximum) |
Intravenous |
2.5 RadioGenix System Maintenance
- For complete system maintenance and use follow the Operator Guide, RadioGenix System 1.2 (SYS-0060) P/N 40010570.
- Install the RadioGenix System in an operating environment which complies with local and national requirements for production of radiopharmaceutical products (ISO Class 8 or better environment as described in USP General Chapter 797 Pharmaceutical Compounding Sterile Preparations).
- The RadioGenix System is only for use by trained personnel.
- Only use potassium molybdate Mo 99, processing reagent, 0.9% Sodium Chloride Injection, USP and other components, including packs supplied by NorthStar Medical Radioisotopes, LLC [ see How Supplied/Storage and Handling (16.1)]
- Table 3 is a summary of RadioGenix System 1.2 scheduled maintenance and protocol actions. Perform all protocols according to the illustrated directions provided in the Operator Guide, RadioGenix System 1.2 (SYS-0060) P/N 40010570.
|
Table 3 RadioGenix System 1.2 Scheduled Maintenance |
|
|
Protocol Frequency |
Action |
|
Initialize System When prompted or as needed (host computer screen will prompt the operator to perform initialization) |
Perform an initialization cycle when the prompted or when RadioGenix System 1.2 is returned to service after a scheduled or unscheduled downtime, such as an interrupted cycle due to equipment or power failure. |
|
Add/Change 0.9% Sodium Chloride Injection USP Every 10 elutions or 24 hours |
Replace the 0.9% Sodium Chloride Injection, USP container and tubing assembly with a new one. Use hydrogen peroxide wipes |
|
Elute Tc-99m Every elution |
Replace the technetium Tc-99m product cartridge, technetium Tc-99m product vial, and the product port caps. Use isopropyl alcohol (IPA) wipes |
|
Add/Change NaOH and PSC Every 10 elutions or after sterilization |
Replace the PSC pack consisting of a primary separation cartridge (PSC) and the tube assembly. Replace the 5M sodium hydroxide 120 mL bottle. Use hydrogen peroxide wipes |
|
Add/Remove Source Vessel No later than expiration date indicated on source veesel |
Replace each potassium molybdate Mo-99 source solution with a new Mo-99 source. Use each potassium molybdate Mo-99 source solution by the indicated expiration date on the label. |
|
Sterilization Weekly |
Perform the software-driven ozonated water system sterilization process Replace the 0.1 micrometer RGX air filter. Use hydrogen peroxide wipes |
|
Exchange Discarded Material Every 200 elutions or earlier |
Remove the radioactive waste (the discarded material container holds 3.5 liters) using appropriate safety measures. Replace with a fresh container. |
2.6 Directions for Eluting RadioGenix System
- The Sodium Pertechnetate Tc 99m Injection solution is produced using Elute Tc-99m protocol through the RadioGenix System home screen Follow step-by-step directions for use provided in the Operator Guide, RadioGenix System 1.2 (SYS-0060) P/N 40010570.
- The elution process to produce Sodium Pertechnetate Tc 99m Injection involves the initial installation and set-up of the equipment, reagent, sterilizing filters, and sterile final product collection vials provided by NorthStar Medical Radioisotopes, LLC [see Table 3].
- Use only isopropyl alcohol (IPA) wipes during the Elution Protocol for the RadioGenix System
- Implement the following prerequisites before the Elute Tc-99m protocol is initiated:
- Connect the potassium molybdate Mo-99 source container using the Source Vessel Kit for RadioGenix System.
- Aseptically install the PSC Pack for RadioGenix System 1.2 consisting of one reagent solution (5M sodium hydroxide NaOH) and the primary separation cartridge (PSC).
- Aseptically assemble and install the Tc-99m Elution Pack for RadioGenix System 1.2 with the TPC consisting of an alumina column, 0.2-micron filter, and a 20 mL sterile collection vial.
- Attach the supplied tubing contained in the Saline Tubing Pack to the saline port.
- Attach the supplied sterile 0.9% sodium chloride injection,USP bag to the saline tubing.
- Initiate the computer-controlled elution process to prepare Sodium Pertechnetate Tc 99m Injection.
- After delivery of Sodium Pertechnetate Tc 99m Injection to the collection vial is complete, remove the collection vial and perform the quality control procedures [see Dosage and Administration (2.7)].
- The first eluate from every Potassium Molybdate Mo-99 Source Vessel may be used for preparation. Discarding of the eluate is not necessary.
2.7 Quality Control of Sodium Pertechnetate Tc 99m Injection
Perform the following quality control procedures on each Sodium Pertechnetate Tc 99m Injection prior to its release for clinical use or for reconstitution with Tc-99m radiopharmaceutical kits.
Mo-99 Breakthrough Test
- Using a suitable radioactivity calibrator, determine the activity of technetium Tc-99m eluted.
- Place the Sodium Pertechnetate Tc-99m injection eluate in a calibrated Mo-99 assay shield. Place the lid on the container and put the entire container in the dose calibrator chamber.
- Record the activity of molybdenum Mo-99 on the most sensitive scale.
- Divide the activity of molybdenum Mo-99 by the activity of technetium Tc-99m. Correct for decay and shielding effect, if necessary.
- Determine the molybdenum Mo-99/technetium Tc-99m ratio at the time of elution and from that ratio, determine the expiration time of the eluate. Each Sodium Pertechnetate Tc 99m Injection eluate must meet the purity requirement of 0.15 microCi of Mo-99 per mCi of Tc-99m.
- The expiry time for each eluate of Sodium Pertechnetate Tc 99m Injection must be no later than 24 hours post-elution or the time where the Mo-99 to Tc-99m ratio reaches 0.15 microCi/mCi, whichever occurs earlier
Colorimetric Aluminum Ion Test Procedure
- Using an aluminum ion indicator kit, determine the aluminum ion concentration of the eluate per the manufacturer's instructions.
- The eluate concentration must not exceed 10 micrograms/mL.
Determination of pH
- Place a small drop of Sodium Pertechnetate Tc-99m injection on a colorimetric pH strip.
- Examine and compare the coloration of the test strip with the colors displayed on the pH cartridge.
- The pH range must be between 4.5 and 7.5.
2.8 Radiolabeling (Reconstitution) of Kits
- In general, use no more than 5 mL volume for radiolabeling kits with RadioGenix System produced Sodium Pertechnetate Tc 99m Injection, USP.
- Perform quality control of a radiolabeled kit per the directions in the package insert and only use the product if it meets the kit manufacturer’s quality control requirements.
- The radiolabeled product shall have an expiry no more than 24 hours from the time of sodium pertechnetate elution or the expiry time stated by the kit manufacturer, whichever occurs earlier.
2.9 Radiation Dosimetry
Intravenous Injection
Estimates of radiation absorbed dose per unit activity of Sodium Pertechnetate Tc 99m Injection administered to an adult of average size and weight and to pediatric patients of sizes and weights typical of representative ages are shown in Table 4.
|
Table 4 Radiation Absorbed Dose from Intravenous Injection |
|||||
|
Age |
Adult |
15 years |
10 years |
5 years |
1 year |
|
Organ |
Absorbed dose per unit activity Sodium Pertechnetate Tc 99m Injection administered intravenously with no thyroid-blocking agent (microGy/MBq)* |
||||
|
Adrenals |
3.7 |
4.6 |
7.1 |
11 |
19 |
|
Bone Surfaces |
5.4 |
6.5 |
9.6 |
14 |
25 |
|
Brain |
2 |
2.5 |
4.1 |
6.5 |
11 |
|
Breasts |
1.8 |
2.3 |
3.4 |
5.6 |
11 |
|
Gallbladder Wall |
7.4 |
9.8 |
16 |
23 |
35 |
|
GI Tract | |||||
|
Esophagus |
2.5 |
3.2 |
4.8 |
7.5 |
14 |
|
Stomach Wall |
26 |
34 |
48 |
78 |
160 |
|
Small Intestine |
16 |
20 |
31 |
47 |
82 |
|
Colon Wall |
41 |
53 |
89 |
140 |
270 |
|
ULI Wall |
56 |
73 |
120 |
200 |
370 |
|
LLI Wall |
21 |
27 |
45 |
71 |
130 |
|
Heart Wall |
3.1 |
4 |
6 |
9.1 |
16 |
|
Kidneys |
5 |
6 |
8.6 |
13 |
21 |
|
Liver** |
4.8 |
6 |
10 |
15 |
28 |
|
Lungs |
2.6 |
3.4 |
5.1 |
7.9 |
14 |
|
Muscles |
3.2 |
4 |
6 |
9.1 |
16 |
|
Ovaries |
9.9 |
13 |
18 |
27 |
44 |
|
Pancreas |
5.6 |
7.2 |
11 |
16 |
27 |
|
Red Marrow |
3.7 |
4.4 |
6.5 |
9 |
15 |
|
Salivary Glands |
8.5 |
10 |
14 |
18 |
26 |
|
Skin |
1.8 |
2.2 |
3.5 |
5.6 |
10 |
|
Spleen |
4.3 |
5.3 |
8 |
12 |
20 |
|
Testes |
2.8 |
3.7 |
5.9 |
9.1 |
16 |
|
Thymus |
2.5 |
3.2 |
4.8 |
7.5 |
14 |
|
Thyroid |
22 |
36 |
54 |
120 |
220 |
|
Urinary Bladder Wall |
18 |
23 |
34 |
45 |
66 |
|
Uterus |
8.1 |
10 |
16 |
23 |
37 |
|
Remaining Tissues |
3.7 |
4.7 |
7.1 |
11 |
19 |
|
Effective dose* per administered activity (microSv/MBq) |
|||||
|
13 |
17 |
26 |
42 |
79 |
|
|
*To obtain radiation absorbed dose per unit activity in mrad/mCi from the preceding table, multiply individual values by a factor of 3.7. (For effective dose per administered activity, the resulting unit is mrem/mCi.) **For the liver, 20% of the absorbed dose per unit activity is derived from a presumed maximum concentration of 0.015% MBq Mo-99 per MBq Tc-99m |
|||||
Dacryoscintigraphy
Estimates of radiation absorbed dose to an adult patient from the nasolacrimal imaging procedure using a maximum dose of 3.7 megabecquerels (0.1 millicurie) of Sodium Pertechnetate Tc 99m Injection are shown in Table 5.
|
Table 5 Radiation Absorbed Dose in the Eye Lens from Dacryoscintigraphy of Adults |
||
|
3.7 MBq (0.1 mCi) of Sodium Pertechnetate Tc-99m |
||
|
(mGy) |
(rad) |
|
|
If lacrimal fluid turnover is 16% per min |
0.14 |
0.014 |
|
If lacrimal fluid turnover is 100% per min |
0.022 |
0.002 |
Cystography
Estimates of radiation absorbed dose per unit activity of Sodium Pertechnetate Tc 99m Injection administered through direct urinary-bladder infusion with no voiding over 30 minutes to an adult of average size and weight and to pediatric patients of sizes and weights typical of representative ages are shown in Table 6.
|
Table 6 Radiation Absorbed Dose* from Cystography |
||||||
|
Age |
Adult |
15 years |
10 years |
5 years |
1 year |
Newborn |
|
Organ |
Absorbed dose per unit activity Sodium Pertechnetate Tc 99m Injection administered through direct urinary-bladder infusion with no voiding over 30 minutes (microGy/MBq) |
|||||
|
Bone Surfaces |
0.19 |
0.24 |
0.35 |
0.51 |
0.95 |
1.8 |
|
Kidneys |
0.035 |
0.051 |
0.11 |
0.22 |
0.37 |
0.83 |
|
Ovaries |
0.97 |
1.2 |
1.8 |
2.6 |
3.9 |
7.1 |
|
Red Marrow |
0.14 |
0.19 |
0.28 |
0.34 |
0.41 |
0.67 |
|
Testes |
0.67 |
0.95 |
1.7 |
2.6 |
4.7 |
8.5 |
|
Urinary Bladder Wall |
20 |
26 |
37 |
55 |
101 |
237 |
|
Effective dose equivalent per administered activity (microSv/MBq) |
||||||
|
1.7 |
2.2 |
3.2 |
4.7 |
8.3 |
19 |
|
|
*To obtain radiation absorbed dose per unit activity in mrad/mCi from the preceding table, multiply individual values by a factor of 3.7. (For effective dose equivalent per administered activity, the resulting unit is mrem/mCi.) |
||||||
Dosage Forms and Strengths
The RadioGenix System provides Sodium Pertechnetate Tc 99m Injection, USP, from a non-highly enriched uranium source of potassium molybdate Mo-99, as a clear, colorless solution containing 4 mCi/mL to 2135 mCi/mL (148 MBq/mL to 78,995 MBq/mL) of technetium Tc 99m radioactivity in approximately 5 mL of volume. The amount of Tc-99m radioactivity depends on the radioactivity in the potassium molybdate Mo-99 source. The source is supplied in vessels containing 7.5 Ci (277.5 GBq), 12 Ci (444 GBq), 15 Ci (555 GBq), and 19 Ci (703 GBq) at the date and time of calibration.
Warnings and Precautions
5.1 Radiation Exposure Risk
Sodium Pertechnetate Tc-99m contributes to a patient's overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Use the lowest dose of Sodium Pertechnetate Tc-99m necessary for imaging and ensure safe handling and preparation to protect the patient and healthcare worker from unintentional radiation exposure. Encourage patients to drink fluids and void as frequently as possible after intravenous or intravesicular administration. Advise patients to blow their nose and wash their eyes with water after ophthalmic administration [see Dosage and Administration (2.1)].
Radiation risks associated with the use of Sodium Pertechnetate Tc-99m are greater in pediatric patients than in adults due to greater absorbed radiation doses and longer life expectancy. Ensure the diagnostic benefit of Sodium Pertechnetate Tc-99m outweighs these greater risks prior to administration in pediatric patients.
5.2 Unintended Mo-99 Exposure
Unintended exposure to Mo-99 radiation contributes to a patient’s overall cumulative radiation dose. To minimize the risk of unintended radiation exposure, strict adherence to the eluate testing protocol is required. Use only potassium molybdate Mo 99, processing reagent, 0.9% Sodium Chloride Injection, USP, and other supplies, including kit and packs, provided by NorthStar Medical Radioisotopes, LLC. Do not administer Sodium Pertechnetate Tc 99m Injection after the 0.15 microCi of Mo 99/mCi of Tc 99m limit has been reached and discard the Sodium Pertechnetate Tc 99m Injection when the 24 hour expiration time is reached, whichever occurs earlier [see Dosage and Administration (2.7)].
5.3 Hypersensitivity Reactions
Hypersensitivity reactions, including serious signs and symptoms of anaphylaxis, following administration of Sodium Pertechnetate Tc 99m Injection have been reported. Always have cardiopulmonary resuscitation equipment and personnel available and monitor all patients for hypersensitivity reactions.
Adverse Reactions/Side Effects
The following adverse reactions are described elsewhere in the labeling:
- Radiation Exposure Risk [see Warnings and Precautions (5.1)]
- Unintended Mo-99 Exposure [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
6.1 Postmarketing Experience
The following adverse reactions associated with the use of Sodium Pertechnetate Tc 99m Injection have been identified in postmarketing experience. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Allergic reactions (skin rash, hives, or itching) including anaphylaxis have been reported following the administration of Sodium Pertechnetate Tc-99m.
Related/similar drugs
Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data with Sodium Pertechnetate Tc-99m use in pregnant women to inform any drug-associated risks of developmental outcomes. Animal reproductive studies have not been conducted with Sodium Pertechnetate Tc-99m. All radiopharmaceuticals, including Sodium Pertechnetate Tc-99m, have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. If considering Sodium Pertechnetate Tc-99m administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes based on the radiation dose from Sodium Pertechnetate Tc-99m and the gestational timing of exposure.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There are limited data available in the published literature on the presence of technetium Tc-99m in human milk. There are no data available on the effects of Sodium Pertechnetate Tc-99m on the breast fed infant or the effects on milk production. Exposure of Sodium Pertechnetate Tc-99m to a breastfed infant can be minimized by temporary discontinuation of breast feeding (see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Sodium Pertechnetate Tc-99m, any potential adverse effects on the breastfed child from Sodium Pertechnetate Tc-99m or from the underlying maternal condition.
Clinical Considerations
To decrease radiation exposure to the breastfed infant, advise a lactating woman to pump and discard breastmilk after the administration of Sodium Pertechnetate Tc-99m for 12 to 24 hours, where the duration corresponds to the typical range of administrated activity, 259 MBq to 925 MBq ( 7 mCi to 25 mCi).
8.4 Pediatric Use
Safety and effectiveness have been established for Sodium Pertechnetate Tc-99m in pediatric patients from birth (term neonates) to 17 years of age for thyroid imaging and for urinary bladder imaging via direct isotopic cystography for the detection of vesicoureteral reflux based on clinical experience. Safety and effectiveness have not been established in pediatric patients for salivary gland imaging or nasolacrimal drainage system imaging. Although dose adjustment based on body size or weight is generally recommended, the administered dose should be adequate to obtain acceptable quality diagnostic information [see Dosage and Administration 2.4]. Radiation risks of Sodium Pertechnetate Tc 99m Injection are greater in pediatric patients than adults [see Warnings and Precautions (5.1)].
8.5 Geriatric Use
Studies on the relationship of age to the effects of Sodium Pertechnetate Tc 99m Injection have not been performed in the geriatric population. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Radiogenix System Description
11.1 Chemical Characteristics
The RadioGenix System provides Sodium Pertechnetate Tc 99m Injection, USP for intravenous use, intravesicular use, ophthalmic use, or for preparing radiopharmaceutical kits. The RadioGenix System uses a non-uranium potassium molybdate Mo-99 source solution to produce Sodium Pertechnetate Tc 99m Injection, USP. The RadioGenix® System uses potassium molybdate Mo-99 sources at an activity of 7.5 Ci (277.5 GBq), 12 Ci (444 GBq), 15 Ci (555 GBq), and 19 Ci (703 GBq) at the date and time of calibration.
Elution of RadioGenix System produces Sodium Pertechnetate Tc-99m (Na99mTcO4) in approximately 5 mL of sterile 0.9% sodium chloride injection, USP solution. The activity of Sodium Pertechnetate Tc-99m produced varies (4 mCi/mL to 2,135 mCi/mL of technetium Tc-99m) and depends on the activity of potassium molybdate Mo-99 present in the source container originally, the decay time since the calibration time, and the elapsed time since the previous Sodium Pertechnetate Tc-99m elution.
Sodium Pertechnetate Tc-99m is an inorganic compound with the formula Na99mTcO4. In solution, Sodium Pertechnetate exists as dissociated Na+ cations and pertechnetate TcO4- anions with the following molecular structure:
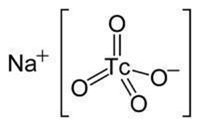



The eluted Sodium Pertechnetate Tc 99m Injection, USP is a sterile, non-pyrogenic, clear, and colorless solution. The pH of the solution is between 4.5 and 7.5.
11.2 Physical Characteristics
Technetium Tc-99m
Technetium Tc-99m decays by isomeric transition with a physical half-life of 6.01 hours. The principal photon that is useful for detection and imaging studies is shown in Table 7.
|
Table 7 Principal Radiation Emission Data technetium Tc-99m |
||
|
Radiation |
Mean Percent Per Disintegration |
Energy (keV) |
|
Gamma-2 |
88.5 |
140.5 |
The air-kerma-rate (exposure-rate) constant for technetium Tc-99m is 5.23 m2·pGy·(MBq)-1·s-1 [0.795 cm2·R·(mCi)-1·h-1]. A range of values for the relative radiation attenuation by the various thicknesses of Pb is shown in Table 8. For example, the use of 3 mm thickness of Pb will attenuate the radiation exposure by a factor of about 1,000.
|
Table 8 Radiation Attenuation by Lead Shielding |
|
|
Shield Thickness (Pb) mm |
Coefficient of Attenuation |
|
0.25 |
0.5 |
|
1 |
10 -1 |
|
2 |
10 -2 |
|
3 |
10 -3 |
|
4 |
10 -4 |
Molybdenum Mo-99
Molybdenum Mo-99 decays to technetium Tc-99m with a molybdenum Mo-99 half-life of 66 hours. This means that 77.7% of the activity remains after 24 hours; 60.4% remains after 48 hours, see Table 9.
|
Table 9 Molybdenum Mo-99 Decay Chart half- life 66.0 hours |
|||
|
Days |
Percent Remaining |
Days |
Percent Remaining |
|
0 * |
100 |
10 |
8 |
|
1 |
77.7 |
11 |
6.3 |
|
2 |
60.4 |
12 |
4.9 |
|
3 |
46.9 |
13 |
3.8 |
|
4 |
36.5 |
14 |
2.9 |
|
5 |
28.4 |
15 |
2.3 |
|
6 |
22 |
20 |
0.6 |
|
7 |
17.1 |
25 |
0.2 |
|
8 |
13.3 |
30 |
0.1 |
|
9 |
10.3 | ||
|
* calibration time |
|||
The physical decay characteristics of molybdenum Mo-99 are such that 88.6% of the decaying molybdenum Mo-99 atoms form Technetium Tc-99m. RadioGenix System elutions may be made at any time, but the amount of technetium Tc-99m available will depend on the time interval measured from the last elution cycle. Eluting the RadioGenix System every 24 hours will provide the maximal yield of Sodium Pertechnetate Tc-99m.
To correct for physical decay of technetium Tc-99m, the fractions that remain at selected intervals of time are shown in Table 10.
|
Table 10 Physical Decay Chart. Technetium Tc-99m, half-life 6.01 Hours |
|||
|
Hours |
Percent Remaining |
Hours |
Percent Remaining |
|
0 * |
100 |
7 |
44.7 |
|
1 |
89.1 |
8 |
39.8 |
|
2 |
79.4 |
9 |
35.5 |
|
3 |
70.8 |
10 |
31.6 |
|
4 |
63.1 |
11 |
28.2 |
|
5 |
56.2 |
12 |
25.1 |
|
6 |
50.1 | ||
|
* calibration time |
|||
Radiogenix System - Clinical Pharmacology
12.1 Mechanism of Action
The pertechnetate ion distributes in the body similarly to the iodide ion, but is not organified. In contrast to the iodide ion, the pertechnetate is released unchanged from the thyroid gland.
12.2 Pharmacodynamics
Pertechnetate concentrates in the thyroid gland, salivary glands, gastric mucosa and choroid plexus. After intravenous administration, it equilibrates with the extracellular space.
Following the administration of Sodium Pertechnetate Tc-99m as an eye drop, the drug mixes with tears within the conjunctival space. Within seconds to minutes it leaves the conjunctival space and escapes into the inferior meatus of the nose through the nasolacrimal drainage system. During this process the pertechnetate ions pass through the canaliculi, the lacrimal sac, and the nasolacrimal duct. In the event of any anatomical or functional blockage of the drainage system there will be a backflow resulting in tearing (epiphora). Thus, the pertechnetate escapes the conjunctival space in the tears. The majority of the pertechnetate escapes within a few minutes of normal drainage and tearing.
12.3 Pharmacokinetics
Times to peak concentrations of pertechnetate following intravenous administration are 3.5 hours for cerebral spinal fluid (CSF) and 0.25 to 2 hours for thyroid (euthyroid patients).
The disappearance of pertechnetate from plasma is biexponential with an initial phase of 10 minutes and a terminal phase of 3 hours. The corresponding phases in CSF are less than 1 hour and 11-12 hours, respectively.
Distribution: Pertechnetate distributes throughout the body concentrating in the gastric mucosa, thyroid gland, salivary glands, and urinary bladder.
Elimination:
Excretion: Elimination by urinary route is 27% in 1 day, 31% in 4 days, and 34% in 8 days based on rate of excretion
Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies have been performed to evaluate carcinogenic potential, mutagenicity potential, or to determine whether Sodium Pertechnetate Tc 99m Injection may affect fertility in males or females.
How is Radiogenix System supplied
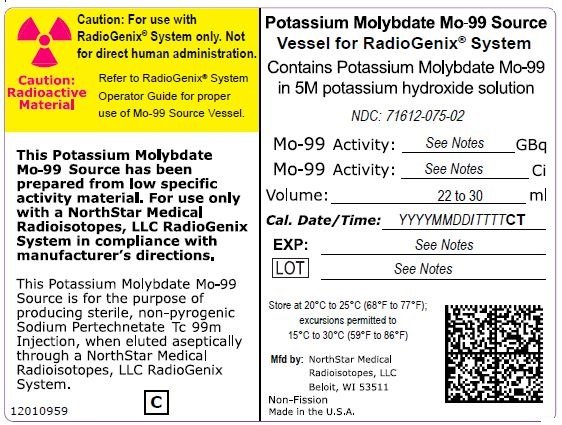
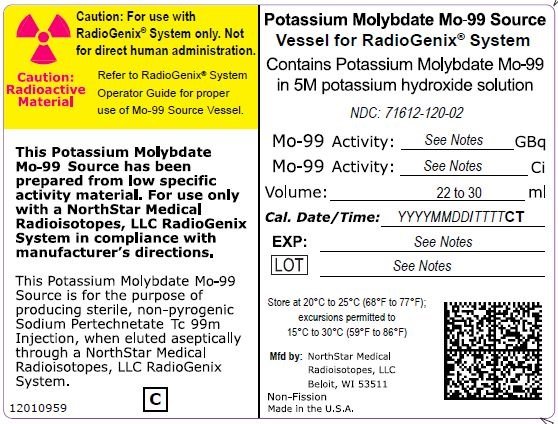
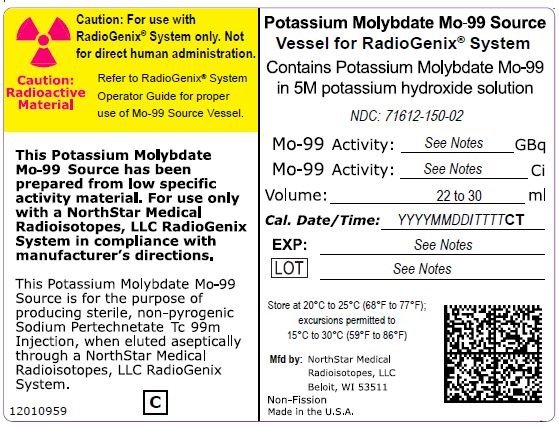
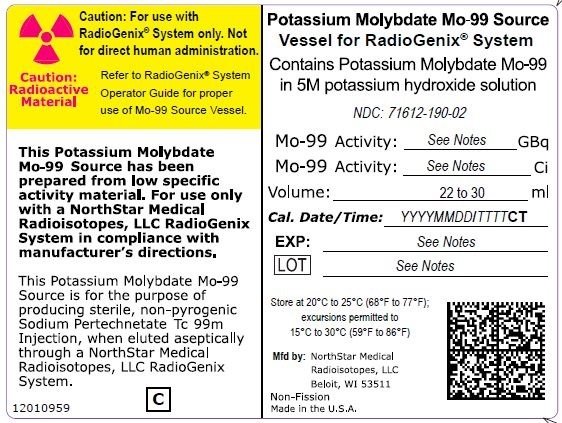
The RadioGenix System is a technetium Tc 99m generator supplied and installed by NorthStar Medical Radioisotopes, LLC. It produces Sodium Pertechnetate Tc 99m Injection, USP from a non-uranium potassium molybdate Mo-99 source solution. The potassium molybdate Mo-99 source solution is shielded within a source container which completely encases a vial that contains 29 mL of solution. NorthStar supplies potassium molybdate Mo-99 solution with the referenced calibration date and time specified on the container label (Table 11).
|
Table 11 Potassium Molybdate Mo-99 Solution Containers |
|||
|
Mo-99 Activity at Time of Calibration |
Product Number |
NDC Number |
|
|
Curies |
Gigabecquerels |
||
|
7.5 |
277.5 |
40008000-75 |
71612-075-02 |
| 12 | 444 | 40008000-12 | 71612-120-02 |
| 15 | 555 | 40008000-15 | 71612-150-02 |
| 19 | 703 | 40008000-19 | 71612-190-02 |
The following kit and packs and consumables (Tables 12-21) are used in the operation of the RadioGenix System as described in the Operator Guide, RadioGenix System 1.2 (SYS-0060) P/N 40010570.
|
Table 12 Materials Supplied in Source Vessel Kit for RadioGenix System, PN 40P07954 |
||
|
Component Description |
Component Part Number |
Qty. |
|
Catheter |
77010566 |
1 |
|
Air Filter* |
77C01237 |
1 |
|
Manifold |
12D09657 |
1 |
|
Absorbent Cloth |
73C05400 |
1 |
|
Black Cap |
77C01489 |
1 |
|
Female Luer Cap* |
77C05449 |
1 |
|
Male Luer Cap* |
77C05450 |
1 |
|
Only used in the removal of a source vessel |
||
|
Table 13 Materials Supplied in Primary Separation Cartridge (PSC) Pack for RadioGenix System 1.2, PN 40P09451 |
||
|
Component Description |
Component Part Number |
Qty. |
|
Primary Separation Cartridge (PSC)* |
40P09852 |
1 |
|
Hydrogen Peroxide Wipe* |
16C07455 |
4 |
|
Tubing Assembly* |
77P09748 |
1 |
|
Table 14 Materials Supplied in Elution Pack for RadioGenix
|
||
|
Component Description |
Component Part Number |
Qty. |
|
Tc-99m Product Cartridge (TPC)* |
40P09853 |
1 |
|
Tc-99m Collection Vial |
77C01318 |
1 |
|
Product Port Cap* |
77C05449 |
1 |
|
Alcohol Wipe* |
16C02704 |
2 |
|
Tc-99m Collection Vial Shield Label |
53D09964 |
1 |
| Tc-99m Collection Vial Label | 53D06431 | 1 |
|
Table 15 Materials Supplied in Sterilization Pack for RadioGenix
|
||
|
Component Description |
Component Part Number |
Qty. |
|
Blank Primary Separation Cartridge (PSC)* |
40P09749 |
1 |
|
Blank Tc-99m Product Cartridge (TPC)* |
40P09850 |
1 |
|
Air Filter* |
77C01237 |
1 |
|
Product Port Cap* |
77C05449 |
1 |
|
Luer Plugs |
77C05450 |
3 |
|
Purge Water Container |
77C05585 |
1 |
|
Hydrogen Peroxide Wipe* |
16C07455 |
9 |
|
Product Vial Label |
53D06430 |
1 |
|
Tubing Assembly |
77P09800 |
1 |
|
Product Vial |
77C01318 |
1 |
|
Table 16 Materials Supplied in Discarded Material Pack for RadioGenix
|
||
|
Component Description |
Component Part Number |
Qty. |
|
Discarded Material Container |
12D05146 |
1 |
|
Silicone Tubing |
77C05431 |
1 |
|
Luer Cap* |
77C05449 |
1 |
| Table 17 Materials Supplied in Discarded Material Type A Pack for RadioGenix System 1.2, PN 40P09855 | ||
| Component Description | Component Part Number | Qty. |
| Discarded Material Container | 12D05146 | 1 |
| Silicone Tubing | 77C05431 | 1 |
| Luer Cap* | 77C05449 | 1 |
| DMC Return Pack | 40P10086 | 1 |
| Table 18 Materials Supplied in Saline Tubing Pack for RadioGenix System 1.2, PN 40P09453 | ||
| Component Description | Component Part Number | Qty. |
|
Hydrogen Peroxide Wipe* | 16C07455 | 1 |
| Saline Tubing* | 77P09747 | 1 |
The following consumables are shipped in bulk to the customer from NorthStar:
|
Table 19 SWFI for RadioGenix System 1.2, PN 16C04488 |
||
|
Component Description |
Component Part Number |
Qty. |
|
Sterile Water for Injection, Bag, 250 mL or 500 mL* |
16C04488 |
24 |
|
Table 20 NaOH for RadioGenix System 1.2 |
||
|
Component Description |
Component Part Number |
Qty. |
|
5M Sodium Hydroxide (NaOH), NF, 120 mL* |
16P09302 |
6 |
| 5M Sodium Hydroxide (NaOH), NF, 120mL* | 35010578 | 6 |
|
Table 21 0.9% Sodium Chloride Injection, USP for RadioGenix System 1.2, PN 16C09849 |
||
|
Component Description |
Component Manufacturer NDC |
Qty |
|
0.9% Sodium Chloride Injection, USP, 250 mL Bag* |
0264-7800-20 |
12, 24, 36 |
|
0.9% Sodium Chloride Injection, USP, 500 mL Bag* |
0264-7800-10 |
12, 24, 36 |
|
0.9% Sodium Chloride Injection, USP, 250 mL Bag* |
0338-0049-02 |
12, 24, 36 |
|
0.9% Sodium Chloride Injection, USP, 500 mL Bag* |
0338-0049-03 |
12, 24, 36 |
|
0.9% Sodium Chloride Injection, USP, 250 mL Bag* |
0409-7983-02 |
12, 24, 36 |
|
0.9% Sodium Chloride Injection, USP, 500 mL Bag* |
0409-7983-03 |
12, 24, 36 |
*Indicates sterile components
Storage and Handling
16.2 Storage and Handling
Storage
- Receipt, transfer, storage, handling, possession, or use of the potassium molybdate Mo-99 source solution, Sodium Pertechnetate Tc 99m Injection, and radioactive components of the RadioGenix System are subject to the radioactive material regulations and licensing requirements of the U.S. Nuclear Regulatory Commission, Agreement States, or Licensing States.
- Install and operate RadioGenix System 1.2, and store the potassium molybdate Mo-99 source solutions, reagent, kit and packs [Sterilization Pack for RadioGenix System 1.2 (PN 40P09444), PSC Pack for RadioGenix System 1.2 (PN 40P09451), Elution Pack for RadioGenix System 1.2 (PN 40P09452), Discarded Material Pack for RadioGenix System 1.2 (PN 40P09855), Source Vessel Kit for RadioGenix System (PN 40P07954), and Saline Tubing Pack Assembly for RadioGenix System 1.2 (PN 40P09453), NaOH for RadioGenix System 1.2 (PN 16P09302) and 0.9% Sodium Chloride Injection, USP (PN 16C09849) at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Disposal
- The maximum use period of a RadioGenix System and ozone generator is one year from the date of installation. After expiry, have NorthStar perform annual preventative maintenance and recertify the RadioGenix System.
- When the potassium molybdate Mo-99 source has reached the end of its useful life or expiration date, remove the source vessel from the RadioGenix System and return it to NorthStar for processing.
- Dispose of the radioactive waste (discarded material) container in accordance with applicable regulations.
PATIENT COUNSELING INFORMATION for RadioGenix System 1.2
Administration Instructions:
Intravenous or Intravesicular Administration
Advise patients to hydrate before (4 hours) and after administration and to void as soon as the imaging study is completed and as often as possible thereafter for the next 12 hours to minimize radiation exposure [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)].
Ophthalmic Administration
After the termination of the nasolacrimal imaging procedure, advise patient to blow their nose and/or wash their eyes with sterile distilled water to further minimize the radiation dose [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)].
Pregnancy:
Advise pregnant women of the risk of fetal exposure to radiation dose if they undergo a radionuclide procedure [see Use in Specific Populations (8.1)].
Lactation:
Advise a lactating woman that exposure of the infant to technetium Tc-99m through breast milk can be minimized if breastfeeding is interrupted when technetium Tc-99m is administered. Advise a lactating woman to pump and discard breast milk for 12 to 24 hours based on the injected dose [see Use in Specific Populations (8.2)].
Manufactured and Distributed by:
NorthStar Medical Radioisotopes, LLC
1800 Gateway Blvd
Beloit, Wisconsin 53511
SYS-0051 Rev 08
| RADIOGENIX SYSTEM
technetium tc 99m generator injection |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| RADIOGENIX SYSTEM
technetium tc 99m generator injection |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| RADIOGENIX SYSTEM
technetium tc 99m generator injection |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| RADIOGENIX SYSTEM
technetium tc 99m generator injection |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - NorthStar Medical Radioisotopes, LLC (025677872) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NorthStar Medical Radioisotopes, LLC | 080328423 | api manufacture(71612-075, 71612-120, 71612-150, 71612-190) , pack(71612-075, 71612-120, 71612-150, 71612-190) , label(71612-075, 71612-120, 71612-150, 71612-190) | |