Imcivree: Package Insert / Prescribing Info
Package insert / product label
Generic name: setmelanotide
Dosage form: subcutaneous injection
Drug class: Melanocortin receptor agonists
Medically reviewed by Drugs.com. Last updated on May 26, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
IMCIVREE® (setmelanotide) injection, for subcutaneous use
Initial U.S. Approval: 2020
Recent Major Changes
Indications and Usage for Imcivree
IMCIVREE is a melanocortin 4 (MC4) receptor agonist indicated to reduce excess body weight and maintain weight reduction long term in adults and pediatric patients 2 years of age and older with syndromic or monogenic obesity due to:
- Bardet-Biedl syndrome (BBS). ( 1)
- Pro-opiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency as determined by an FDA-approved test demonstrating variants in POMC, PCSK1, or LEPRgenes that are interpreted as pathogenic, likely pathogenic, or of uncertain significance (VUS). ( 1)
Limitations of Use:
IMCIVREE is notindicated for the treatment of patients with the following conditions as IMCIVREE would not be expected to be effective:
- Obesity due to suspected POMC, PCSK1, or LEPR-deficiency with POMC, PCSK1, or LEPRvariants classified as benign or likely benign. ( 1)
- Other types of obesity not related to BBS or POMC, PCSK1 or LEPR deficiency, including obesity associated with other genetic syndromes and general (polygenic) obesity. ( 1)
Imcivree Dosage and Administration
- Select patients for treatment who have a clinical diagnosis of BBS or who have genetically determined or suspected deficiency of POMC, PCSK1, or LEPR. ( 2.1)
- Recommended starting dosage injected subcutaneously for:
- Recommended maintenance dosage for adults and pediatric patients aged 6 years and older is 3 mg (0.3 mL) injected subcutaneously once daily. ( 2.2, 2.3)
- Recommended maintenance dose for pediatric patients aged 2 to less than 6 years is determined by body weight. ( 2.4)
- For recommended dosage in patients with renal impairment, see Full Prescribing Information. ( 2.5)
- For titration and administration recommendations, see Full Prescribing Information. ( 2.2, 2.3, 2.4, 2.5, 2.6)
Dosage Forms and Strengths
Injection: 10 mg/mL solution in a 1 mL multiple-dose vial for single patient use ( 3)
Contraindications
Prior serious hypersensitivity to setmelanotide or any of the excipients in IMCIVREE ( 4)
Warnings and Precautions
- Disturbance in Sexual Arousal:Spontaneous penile erections in males and sexual adverse reactions in females have occurred. Inform patients that these events may occur and instruct patients who have an erection lasting longer than 4 hours to seek emergency medical attention. ( 5.1)
- Depression and Suicidal Ideation:Depression and suicidal ideation have occurred. Monitor patients for new onset or worsening depression or suicidal thoughts or behaviors. Consider discontinuing IMCIVREE if patients experience suicidal thoughts or behaviors, or clinically significant or persistent depression symptoms occur. ( 5.2)
- Hypersensitivity Reactions:Serious hypersensitivity reactions (e.g., anaphylaxis) have been reported. If suspected, advise patients to promptly seek medical attention and discontinue IMCIVREE. ( 5.3).
- Skin Hyperpigmentation, Darkening of Pre-Existing Nevi, and Development of New Melanocytic Nevi:Generalized increased skin pigmentation, darkening of pre-existing nevi, and development of new nevi have occurred. Perform a full body skin examination prior to initiation and periodically during treatment to monitor pre-existing and new pigmentary lesions. ( 5.4)
- Risk of Serious Adverse Reactions Due to Benzyl Alcohol Preservative in neonates and low birth weight infants:_IMCIVREE is not approved for use in neonates or infants. Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and low birth weight infants treated with benzyl alcohol-preserved drugs. ( 5.5)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥20%) included skin hyperpigmentation, injection site reactions, nausea, headache, diarrhea, abdominal pain, vomiting, depression, and spontaneous penile erection. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Rhythm Pharmaceuticals at 1-833-789-6337 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- Lactation:not recommended when breastfeeding ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2025
Full Prescribing Information
1. Indications and Usage for Imcivree
IMCIVREE is indicated to reduce excess body weight and maintain weight reduction long term in adults and pediatric patients aged 2 years and older with syndromic or monogenic obesity due to:
- Bardet-Biedl syndrome (BBS) [see Dosage and Administration ( 2.1)]
- Pro-opiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency as determined by an FDA-approved test demonstrating variants in POMC, PCSK1, or LEPRgenes that are interpreted as pathogenic, likely pathogenic, or of uncertain significance (VUS) [see Dosage and Administration ( 2.1)].
Limitations of Use:
IMCIVREE is
notindicated for the treatment of patients with the following conditions as
IMCIVREE would not be expected to be effective:
- Obesity due to suspected POMC, PCSK1, or LEPR deficiency with POMC, PCSK1, or LEPRvariants classified as benign or likely benign
- Other types of obesity not related to BBS or POMC, PCSK1, or LEPR deficiency, including obesity associated with other genetic syndromes and general (polygenic) obesity
2. Imcivree Dosage and Administration
2.1 Patient Selection
BBS
- Select patients for treatment with IMCIVREE who have a clinical diagnosis of BBS [see Clinical Studies ( 14)]. Consider genetic confirmation in pediatric patients aged <6 years.
POMC, PCSK1, or LEPR Deficiency
- Select patients for treatment with IMCIVREE who have genetically determined or suspected deficiency of POMC, PCSK1, or LEPR [see Clinical Studies ( 14)].
- Treat patients with variants in POMC, PCSK1, or LEPR genes that are interpreted as pathogenic, likely pathogenic, or of uncertain significance (VUS) in the clinical context of the patient [see Clinical Studies ( 14)].
- Information on an FDA-approved test for the detection of variants in the POMC, PCSK1,or LEPRis available at http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage in Adults and Pediatric Patients Aged 12 Years and Older
- The recommended starting dosage is 2 mg (0.2 mL) injected subcutaneously once daily for 2 weeks in adults and pediatric patients aged 12 years and older.
- Monitor patients for gastrointestinal (GI) adverse reactions during dosage initiation and titration [see Adverse Reactions ( 6.1)] .
- If the starting dosage is:
- Not tolerated, reduce the dosage to 1 mg (0.1 mL) once daily. If the 1 mg once daily dosage is tolerated for at least 1 week, increase the dosage to 2 mg (0.2 mL) once daily.
- Tolerated for 2 weeks, increase the dosage to 3 mg (0.3 mL) once daily. If the 3 mg once daily dosage is not tolerated, decrease the dosage to 2 mg (0.2 mL) once daily.
- The recommended maintenance dosage is 3 mg (0.3 mL) injected subcutaneously once daily.
2.3 Recommended Dosage in Pediatric Patients Aged 6 to Less Than 12 Years
- The recommended starting dosage is 1 mg (0.1 mL) injected subcutaneously once daily for 2 weeks in pediatric patients aged 6 to less than 12 years.
- Monitor patients for GI adverse reactions during dosage initiation and titration [see Adverse Reactions ( 6.1)] .
- If the starting dosage is:
- Not tolerated, reduce the dosage to 0.5 mg (0.05 mL) once daily. If the 0.5 mg once daily dosage is tolerated for at least 1 week, increase the dosage to 1 mg (0.1 mL) once daily.
- Tolerated for 2 weeks, increase the dosage to 2 mg (0.2 mL) once daily. If the 2 mg daily dosage is:
- Not tolerated, reduce the dosage to 1 mg (0.1 mL) once daily.
- Tolerated, increase the dosage to 3 mg (0.3 mL) once daily.
- The recommended maintenance dosage is 3 mg (0.3 mL) injected subcutaneously once daily.
2.4 Recommended Dosage in Pediatric Patients Aged 2 to Less Than 6 Years
- The recommended starting dosage is 0.5 mg (0.05 mL) injected subcutaneously once daily for 2 weeks in pediatric patients aged 2 to less than 6 years.
- Monitor patients for GI adverse reactions during dosage initiation and titration [see Adverse Reactions ( 6.1)] .
- If the starting dosage is:
- Not tolerated, discontinue the product.
- Tolerated for 2 weeks, increase the dosage based on baseline body weight, as presented in Table 1.
| Patient Weight/Treatment Week | Daily Dose | Volume to be Injected |
| 15 kg to less than 20 kg | ||
| Week 1 and onward | 0.5 mg once daily | 0.05 mL once daily |
| 20 kg to less than 30 kg | ||
| Weeks 1‑2 | 0.5 mg once daily | 0.05 mL once daily |
| Week 3 and onward | 1 mg once daily | 0.1 mL once daily |
| 30 kg to less than 40 kg | ||
| Weeks 1‑2 | 0.5 mg once daily | 0.05 mL once daily |
| Weeks 3‑4 | 1 mg once daily | 0.1 mL once daily |
| Week 5 and onward | 1.5 mg once daily | 0.15 mL once daily |
| Greater than or equal to 40 kg | ||
| Weeks 1‑2 | 0.5 mg once daily | 0.05 mL once daily |
| Weeks 3‑4 | 1 mg once daily | 0.1 mL once daily |
| Weeks 5‑6 | 1.5 mg once daily | 0.15 mL once daily |
| Weeks 7 and onward | 2 mg once daily | 0.2 mL once daily |
2.5 Recommended Dosage in Patients with Renal Impairment
Recommended Dosage in Adults and Pediatric Patients Aged 2 Years and Older with End Stage Renal Disease [estimated glomerular filtration (eGFR) less than 15 mL/min/1.73 m 2]
IMCIVREE is not recommended for use in patients with end stage renal disease.
Recommended Dosage in Patients with Severe Renal Impairment (eGFR of 15 to 29 mL/min/1.73 m 2)
Adults and Pediatric Patients Aged 12 Years and Older
- The recommended starting dosage is 0.5 mg (0.05 mL) injected subcutaneously once daily for 2 weeks in adults and pediatric patients aged 12 years and older with severe renal impairment.
- Monitor patients for GI adverse reactions during dosage initiation and titration [see Adverse Reactions ( 6.1)] .
- If the recommended starting dosage is
[see Use in Specific Populations (
8.6)]
:
- Not tolerated, discontinue IMCIVREE.
- Tolerated for 2 weeks, increase the dosage to 1 mg (0.1 mL) once daily. If the 1 mg daily dosage is tolerated for at least 1 week, increase the dosage to 1.5 mg (0.15 mL) once daily. The recommended maintenance dosage is 1.5 mg (0.15 mL) injected subcutaneously once daily [see Use in Specific Populations ( 8.6) and Clinical Pharmacology ( 12.3)] .
Pediatric Patients Ages 6 Years to Less Than 12 Years
- The recommended starting dosage is 0.5 mg (0.05 mL) injected subcutaneously once daily for 2 weeks in pediatric patients aged 6 to less than 12 years with severe renal impairment.
- Monitor patients for GI adverse reactions during dosage initiation and titration [see Adverse Reactions ( 6.1)] .
- If the recommended starting dosage is
[see Use in Specific Populations (
8.6)]
:
- Not tolerated, discontinue IMCIVREE.
- Tolerated for 2 weeks, increase the dosage to 1 mg (0.1 mL) injected subcutaneously once daily. The recommended maintenance dosage is 1 mg (0.1 mL) injected subcutaneously once daily
Pediatric Patients Aged 2 to Less Than 6 Years Weighing at Least 20 kg
- The recommended starting dosage is 0.5 mg (0.05 mL) injected subcutaneously once daily for 2 weeks in pediatric patients aged 2 to less than 6 years with severe renal impairment and weight of at least 20 kg.
- Monitor patients for GI adverse reactions during dosage initiation and titration [see Adverse Reactions ( 6.1)] .
- If the recommended starting dosage is [see Use in Specific Populations ( 8.6)] :
| Patient Weight/Treatment Week | Daily Dose | Volume to be Injected |
| 20 kg to less than 30 kg | ||
| Week 1 and onward | 0.5 mg once daily | 0.05 mL once daily |
| 30 kg to less than 40 kg | ||
| Weeks 1‑2 | 0.5 mg once daily | 0.05 mL once daily |
| Week 3 and onward | 1 mg once daily | 0.1 mL once daily |
| Greater than or equal to 40 kg | ||
| Weeks 1‑2 | 0.5 mg once daily | 0.05 mL once daily |
| Week 3 and onward | 1 mg once daily | 0.1 mL once daily |
- The recommended maintenance dosage is 1 mg (0.1 mL) injected subcutaneously once daily [see Use in Specific Populations ( 8.6) and Clinical Pharmacology ( 12.3)] . Monitor patients for adverse reactions [see Adverse Reactions ( 6.1)] .
Recommended Dosage in Adults and Pediatric Patients Aged 2 Years and Older with Mild (eGFR of 60 to 89 mL/min/1.73 m 2) or Moderate (eGFR of 30 to 59 mL/min/1.73 m 2) Renal Impairment
The recommended dosage in patients with mild or moderate renal impairment is the same as in those with normal kidney function [see Dosage and Administration ( 2.2, 2.3, 2.4)] .
2.6 Administration Instructions
- Prior to initiation of IMCIVREE, train patients or their caregivers on proper injection technique. Instruct patients to use a 1-mL syringe with a 28-gauge or 29-gauge needle appropriate for subcutaneous injection.
- Remove IMCIVREE from the refrigerator approximately 15 minutes prior to administration. Alternatively, warm IMCIVREE prior to administration by rolling the vial gently between the palms of the hands for 60 seconds.
- Inspect IMCIVREE visually before use. It should appear clear to slightly opalescent, colorless to slightly yellow. Do not use if particulate matter or discoloration is seen.
- Administer IMCIVREE once daily, at the beginning of the day, without regard to meals.
- Inject IMCIVREE subcutaneously in the abdomen, thigh, or arm, rotating to a different site each day. Do not administer IMCIVREE intravenously or intramuscularly.
- If a dose is missed, resume the once daily regimen as prescribed with the next scheduled dose.
3. Dosage Forms and Strengths
Injection: 10 mg/mL, clear to slightly opalescent, colorless to slightly yellow solution in a 1-mL multiple-dose vial for single patient use.
4. Contraindications
IMCIVREE is contraindicated in patients with a prior serious hypersensitivity reaction to setmelanotide or any of the excipients in IMCIVREE. Serious hypersensitivity reactions have included anaphylaxis [see Warnings and Precautions ( 5.3)].
5. Warnings and Precautions
5.1 Disturbance in Sexual Arousal
Sexual adverse reactions may occur in patients treated with IMCIVREE. Spontaneous penile erections in males (24% in patients aged 6 years and older; 8% in patients aged 2 to less than 6 years) and sexual adverse reactions in females (7% in IMCIVREE-treated patients aged 6 years and older and 0% in placebo-treated patients from an unapproved population) occurred in clinical studies with IMCIVREE [see Adverse Reactions ( 6.1)].
Inform patients that these events may occur and instruct patients who have an erection lasting longer than 4 hours to seek emergency medical attention.
5.2 Depression and Suicidal Ideation
Some drugs that target the central nervous system, such as IMCIVREE, may cause depression or suicidal ideation. Depression (26% in patients aged 6 years and older), suicidal ideation (11% in patients aged 6 years and older), and depressed mood (8% in patients aged 2 to less than 6 years) occurred in adults and pediatric patients in IMCIVREE clinical studies [see Adverse Reactions ( 6.1)]. Patients with a history of depression or suicidal ideation may be at increased risk for recurrent episodes while taking IMCIVREE.
Monitor patients for new onset or worsening of depression, suicidal thoughts or behavior, or any unusual changes in mood or behavior. Consider discontinuing IMCIVREE if patients experience suicidal thoughts or behaviors or if clinically significant or persistent depression symptoms occur.
5.3 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, have been reported with IMCIVREE. These reactions generally occurred within minutes to hours after injecting IMCIVREE [see Adverse Reactions ( 6.2)]. If hypersensitivity reactions occur, advise patients to promptly seek medical attention and discontinue use of IMCIVREE. IMCIVREE is contraindicated in patients with a prior serious hypersensitivity reaction to setmelanotide or any of the excipients in IMCIVREE.
5.4 Skin Hyperpigmentation, Darkening of Pre-Existing Nevi, and Development of New Melanocytic Nevi
Generalized or focal increases in skin pigmentation occurred in the majority of patients (67% in patients aged 6 years and older; 83% in patients 2 to less than 6 years) treated with IMCIVREE in clinical trials [see Adverse Reactions ( 6.1) and Clinical Pharmacology ( 12.1)]. This effect is reversible upon discontinuation of the drug.
IMCIVREE may also cause the development of new melanocytic nevi or darkening of pre-existing nevi due to its pharmacologic effect. Development of new melanocytic nevi and darkening or increase in size of existing melanocytic nevi occurred in 16% of patients aged 6 years and older and 33% of patients aged 2 to less than 6 years.
Perform a full body skin examination prior to initiation and periodically during treatment with IMCIVREE to monitor pre-existing and new skin pigmented lesions
5.5 Risk of Serious Adverse Reactions Due to Benzyl Alcohol Preservative in Neonates and Low Birth Weight Infants
IMCIVREE is not approved for use in neonates or infants. Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and low birth weight infants treated with benzyl alcohol-preserved drugs, including IMCIVREE. The “gasping syndrome” is characterized by central nervous system depression, metabolic acidosis, and gasping respirations. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known (IMCIVREE contains 10 mg of benzyl alcohol per mL) [see Use in Specific Populations ( 8.4)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Disturbance in Sexual Arousal [see Warnings and Precautions ( 5.1)]
- Depression and Suicidal Ideation [see Warnings and Precautions ( 5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions ( 5.3)]
- Skin Hyperpigmentation, Darkening of Pre-Existing Nevi, and Development of New Melanocytic Nevi [see Warnings and Precautions ( 5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Bardet-Biedl Syndrome (Adults and Pediatric Patients Aged 6 Years and Older)
The safety of IMCIVREE was evaluated in a clinical study, which included a 14 week, randomized, double-blind, placebo-controlled period followed by a 52-week open-label, treatment period, in 44 patients aged 6 years and older with obesity and a clinical diagnosis of BBS (Study 1) [see Clinical Studies ( 14)]. The study duration was 66 weeks.
During the 14-week placebo-controlled period in Study 1, the most common reported adverse reactions in IMCIVREE-treated patients when compared to placebo-treated patients were hyperpigmentation disorders (67% vs 0%, respectively) and vomiting (11% vs 0%, respectively).
Adverse reactions were also evaluated during the 52-week active-treatment period, defined as the period from randomization to Week 52 in patients initially randomized to IMCIVREE, and from Week 14 to Week 66 in patients initially randomized to placebo. Table 3summarizes the adverse reactions that occurred in 2 or more IMCIVREE-treated patients in Study 3 during the 52-week active treatment period.
|
143 patients were treated with at least 1 dose of IMCIVREE; 1 patient initially randomized to placebo withdrew from the study prior to receiving IMCIVREE and is not included 2Includes skin hyperpigmentation, hair color changes, melanoderma 3Includes injection site erythema, pruritis, induration, pain, bruising, edema, reaction, hemorrhage, irritation, mass 4n = 20 male patients 5Includes new melanocytic nevus formation, increased melanocytic nevus size, and darkening of pre-existing melanocytic nevus |
|
| Adverse Reaction | IMCIVREE-treated Patients
N = 43 1 % |
| Skin hyperpigmentation 2 | 63 |
| Injection Site Reactions 3 | 51 |
| Nausea | 26 |
| Spontaneous penile erection 4 | 25 |
| Vomiting | 19 |
| Diarrhea | 14 |
| Melanocytic nevus 5 | 14 |
| Headache | 7 |
| Skin striae | 7 |
| Aggression | 5 |
| Fatigue | 5 |
POMC, PCSK1, and LEPR Deficiency (Adults and Pediatric Patients Aged 6 Years and Older)
The safety of IMCIVREE was evaluated in two 52-week, open-label clinical studies of 27 patients aged 6 years and older with obesity due to POMC, PCSK1, or LEPR deficiency with POMC, PCSK1, or LEPR genes that are interpreted as pathogenic, likely pathogenic, or of uncertain significance (Study 2 and Study 3) [see Clinical Studies ( 14)] .
Table 4summarizes the adverse reactions that occurred in the open-label studies during the first 52 weeks of treatment in 3 or more patients treated with IMCIVREE.
|
1Includes injection site erythema, pruritus, edema, pain, induration, bruising, injection site hypersensitivity, hematoma, nodule, and discoloration 2Includes skin hyperpigmentation, pigmentation disorders, skin discoloration, gingival discoloration 3Includes abdominal pain and upper abdominal pain 4Includes depressed mood 5n = 13 male patients 6Includes new melanocytic nevus formation, increased melanocytic nevus size, and darkening of pre-existing melanocytic nevus |
|
| Adverse Reaction | IMCIVREE-treated Patients
N = 27 % |
| Injection site reaction 1 | 96 |
| Skin hyperpigmentation 2 | 78 |
| Nausea | 56 |
| Headache | 41 |
| Diarrhea | 37 |
| Abdominal pain 3 | 33 |
| Back pain | 33 |
| Fatigue | 30 |
| Vomiting | 30 |
| Depression 4 | 26 |
| Upper respiratory tract infection | 26 |
| Spontaneous penile erection 5 | 23 |
| Arthralgia | 19 |
| Asthenia | 19 |
| Melanocytic nevus 6 | 19 |
| Dizziness | 15 |
| Dry mouth | 15 |
| Dry skin | 15 |
| Insomnia | 15 |
| Vertigo | 15 |
| Alopecia | 11 |
| Chills | 11 |
| Constipation | 11 |
| Influenza-like illness | 11 |
| Muscle spasm | 11 |
| Pain in extremity | 11 |
| Rash | 11 |
| Suicidal ideation | 11 |
POMC, PCSK1, and LEPR Deficiency and BBS (Pediatric Patients Aged 2 to <6 Years)
The safety of IMCIVREE was evaluated in one 52-week, open-label clinical study of 12 patients aged 2 to less than 6 years with obesity due to POMC, PCSK1, or LEPR deficiency with POMC, PCSK1,or LEPRgenes that are interpreted as pathogenic, likely pathogenic, or of uncertain significance, or obesity due to BBS (Study 4) [see Clinical Studies ( 14)] . No patients with PCSK1 were enrolled in the trial.
Table 5summarizes the adverse reactions that occurred in the open-label study during 52 weeks of treatment in 3 or more patients treated with IMCIVREE.
|
1Includes skin hyperpigmentation, ephelides, nail pigmentation, pigmentation lip, skin discoloration, gingival hyperpigmentation 2Includes injection site bruising, pruritus, discoloration, erythema, induration, oedema, pain, urticaria 3Includes new melanocytic nevus formation, increased melanocytic nevus size, and darkening of pre-existing melanocytic nevus |
|
| Adverse Reaction | IMCIVREE-treated Patients
N = 12 % |
| Skin hyperpigmentation 1 | 83 |
| Injection Site Reactions 2 | 67 |
| Vomiting | 58 |
| Nasopharyngitis | 42 |
| Melanocytic nevus 3 | 33 |
| Fall | 33 |
| Fever | 33 |
| Upper respiratory tract infection | 33 |
| Cough | 25 |
| Diarrhea | 25 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of IMCIVREE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hypersensitivity, including anaphylaxis
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Discontinue IMCIVREE when pregnancy is recognized unless the benefits of therapy outweigh the potential risks to the fetus.
IMCIVREE contains the preservative benzyl alcohol. Because benzyl alcohol is rapidly metabolized by a pregnant woman, benzyl alcohol exposure in the fetus is unlikely. However, adverse reactions have occurred in premature neonates and low birth weight infants who received intravenously administered benzyl alcohol-containing drugs [see Warnings and Precautions ( 5.5) and Use in Specific Populations ( 8.4)].
There are no available data with IMCIVREE in pregnant women to inform a drug-associated risk for major birth defects and miscarriage, or adverse maternal or fetal outcomes. For the general US population, weight loss offers no potential benefit to a pregnant woman and may result in fetal harm (see Clinical Considerations) . In animal reproduction studies, setmelanotide subcutaneously administered to pregnant rats from before mating to the end of organogenesis was not teratogenic at doses 11 times the maximum recommended human dose (MRHD) of 3 mg. Setmelanotide subcutaneously administered to pregnant rabbits during the period of organogenesis was not teratogenic at clinical doses. Setmelanotide administered subcutaneously to pregnant rats during organogenesis through lactation did not result in adverse developmental effects at doses 7 times the MRHD ( see Data).
The estimated background risk of birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Disease-Associated Maternal and/or Embryo/Fetal Risks
Maternal obesity increases the risk for congenital malformations, including neural tube defects, cardiac malformations, oral clefts, and limb reduction defects. In addition, weight loss during pregnancy may result in fetal harm including increased risk of small for gestational age. Appropriate weight gain based on pre-pregnancy weight is currently recommended for all pregnant women, including those who are already overweight or have obesity, due to the obligatory weight gain that occurs in maternal tissues during pregnancy.
Animal Data
Embryo-fetal development was evaluated in female rats administered setmelanotide subcutaneously during mating to end of major organogenesis (14 days prior to mating to gestation day 17) at doses of 0.5, 3, and 5 mg/kg/day, resulting in exposures up to 11 times the human exposure at MRHD of 3 mg, based on AUC. Dose-related decreases in maternal food intake and body weight gain were observed during the premating period but not during gestation. No evidence of embryo-fetal toxicity was observed.
Embryo-fetal development was evaluated in pregnant rabbits subcutaneously administered setmelanotide during organogenesis (gestation days 7 to 19) at doses of 0.05, 0.1, and 0.2 mg/kg/day, resulting in clinically relevant exposures at the MRHD, based on AUC. Decreases in maternal food consumption and body weight were observed at all doses. Increases in embryo-fetal resorptions and post-implantation losses were observed at ≥0.1 mg/kg/day in the presence of significant maternal toxicity, and fetal body weights were 7% lower than controls at 0.2 mg/kg/day.
Pre- and post-natal development was evaluated in rats subcutaneously administered setmelanotide during organogenesis and continuing until weaning (gestation day 6 to lactation day 21) at doses of 0.5, 3.0, and 5.0 mg/kg/day, which resulted in exposures up to 7 times the human exposure at the MRHD, based on AUC. Pup body weights at birth were 9% lower than controls at 3.0 and 5.0 mg/kg/day, which was consistent with reduced maternal body weight gain and food consumption during gestation. No adverse setmelanotide-related effects on pup survival, growth, maturation, visual function, neurobehavioral performance, or reproductive performance were observed up to the highest dose.
8.2 Lactation
Risk Summary
Treatment with IMCIVREE is not recommended for use while breastfeeding.
IMCIVREE from multiple-dose vials contains the preservative benzyl alcohol. Because benzyl alcohol is rapidly metabolized by a lactating woman, benzyl alcohol exposure in the breastfed infant is unlikely. However, adverse reactions have occurred in premature neonates and low birth weight infants who received intravenously administered benzyl alcohol-containing drugs [see Warnings and Precautions ( 5.5) and Use in Specific Populations ( 8.4)] .
There is no information on the presence of setmelanotide or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. However, setmelanotide is present in the milk of rats ( see Data). When a drug is present in rat milk, it is likely that the drug will be present in human milk.
Dose-related setmelanotide concentrations were observed in milk 2 hours after subcutaneous injection in the preweaning phase of a pre- and post-natal development study in rats. No quantifiable setmelanotide concentrations were detected in plasma from nursing pups on post-natal Day 11.
8.4 Pediatric Use
The safety and effectiveness of IMCIVREE have been established to reduce excess body weight and maintain weight reduction long term in pediatric patients aged 2 years and older with obesity due to:
- BBS [see Clinical Studies ( 14.1)]
- POMC, PCSK1, or LEPR deficiency with variants in POMC, PCSK1,or LEPRgenes that are interpreted as pathogenic, likely pathogenic, or of uncertain significance (VUS) [see Clinical Studies ( 14.2)]
Use of IMCIVREE for these indications is supported by evidence from one 66-week study, which included a 14-week, randomized, double-blind, placebo-controlled period followed by a 52-week open-label period, and included 22 pediatric patients with BBS aged 6 to 17 years (Study 1); from two 1-year, open-label studies that included 9 pediatric patients with POMC, PCSK1, or LEPR deficiency aged 6 to 17 years (Study 2 and Study 3); and one 1-year, open-label study that included 12 pediatric patients with POMC or LEPR deficiency or BBS aged 2 to less than 6 years (Study 4) [see Clinical Studies ( 14.1, 14.2, 14.3)] .
Adverse reactions with IMCIVREE treatment in pediatric patients aged 2 to less than 6 years were generally similar to those reported in adults and in pediatric patients aged 6 years and older. Pediatric patients treated with IMCIVREE had greater incidences of vomiting, skin hyperpigmentation, and new or darkening nevi compared to adults treated with IMCIVREE [see Adverse Reactions ( 6.1)] . Perform a full body skin examination prior to initiation and periodically during treatment with IMCIVREE to monitor pre-existing and new skin pigmented lesions. [see Warnings and Precautions ( 5.4)]
The safety and effectiveness of IMCIVREE have not been established in pediatric patients younger than 2 years of age.
IMCIVREE is not approved for use in neonates or infants. Serious adverse reactions including fatal reactions and the “gasping syndrome” occurred in premature neonates and low birth weight infants in the neonatal intensive care unit who received drugs containing benzyl alcohol as a preservative. In these cases, benzyl alcohol dosages of 99 to 234 mg/kg/day produced high levels of benzyl alcohol and its metabolites in the blood and urine (blood levels of benzyl alcohol were 0.61 to 1.378 mmol/L). Additional adverse reactions included gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Preterm, low-birth weight infants may be more likely to develop these reactions because they may be less able to metabolize benzyl alcohol. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known (IMCIVREE contains 10 mg of benzyl alcohol) [see Warnings and Precautions ( 5.5)] .
8.5 Geriatric Use
Clinical studies of IMCIVREE did not include patients aged 65 and over. It is not known whether geriatric patients would respond differently than younger adult patients.
8.6 Renal Impairment
Patients with severe renal impairment have a higher exposure of setmelanotide relative to patients with normal kidney function. Reduce the recommended starting and maintenance dosage of IMCIVREE in adults and pediatric patients 2 years of age and older with severe renal impairment (eGFR 15 29 mL/min/1.73 m 2). The use of IMCIVREE in pediatric patients aged 2 to less than 6 years with weight less than 20 kg and severe renal impairment is not recommended [see Dosage and Administration ( 2.5), Clinical Pharmacology ( 12.3)].
The recommended dosage in patients with mild (eGFR of 60-89 mL/min/1.73 m 2) or moderate renal impairment (eGFR of 30-59 mL/min/1.73 m 2) is the same as those with normal kidney function [see Clinical Pharmacology ( 12.3)].
IMCIVREE is not recommended for use in patients with end stage renal disease (eGFR less than 15 mL/min/1.73 m 2).
10. Overdosage
In the event of an overdose initiate appropriate supportive treatment according to the patient’s clinical signs and symptoms.
11. Imcivree Description
IMCIVREE contains setmelanotide acetate, a melanocortin 4 (MC4) receptor agonist. Setmelanotide is an 8 amino acid cyclic peptide analog of endogenous melanocortin peptide α-MSH (alpha-melanocyte stimulating hormone).
The chemical name for setmelanotide acetate is acetyl-L-arginyl-L-cysteinyl-D-alanyl-L-histidinyl-D-phenylalanyl-L-arginyl-L-tryptophanyl-L-cysteinamide cyclic (2→8)-disulfide acetate. Its molecular formula is C 49H 68N 18O 9S 2(anhydrous, free-base), and molecular mass is 1117.3 Daltons (anhydrous, free-base).
The chemical structure of setmelanotide acetate is:
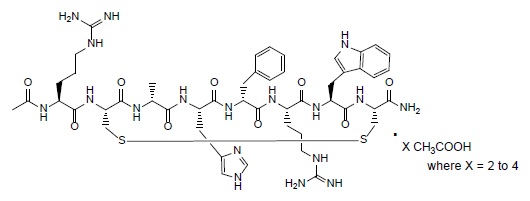
IMCIVREE (setmelanotide) injection is a sterile clear to slightly opalescent, colorless to slightly yellow solution for subcutaneous use. Each 1 mL single-patient use vial of IMCIVREE contains 10 mg of setmelanotide provided as setmelanotide acetate, which is a salt with 2 to 4 molar equivalents of acetate, and the following inactive ingredients: 10 mg benzyl alcohol, 8 mg carboxymethylcellulose sodium (average MWt 90,500), 1 mg edetate disodium dihydrate, 100 mg N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl- glycero-3- phosphoethanolamine sodium salt, 11 mg mannitol, 5 mg phenol, hydrochloric acid, sodium hydroxide and Water for Injection. The pH of IMCIVREE is 5 to 6.
12. Imcivree - Clinical Pharmacology
12.1 Mechanism of Action
Setmelanotide is an MC4 receptor agonist with 20-fold less activity at the melanocortin 3 (MC3) and melanocortin 1 (MC1) receptors. MC4 receptors in the brain are involved in regulation of hunger, satiety, and energy expenditure. Based on nonclinical evidence, setmelanotide may re-establish MC4 receptor pathway activity to reduce food intake and promote weight loss through decreased caloric intake and increased energy expenditure in patients with obesity due to BBS or POMC, PCSK1, or LEPR deficiency associated with insufficient activation of the MC4 receptor. The MC1 receptor is expressed on melanocytes, and activation of this receptor leads to accumulation of melanin and increased skin pigmentation independently of ultraviolet light [see Warnings and Precautions ( 5.4) and Adverse Reactions ( 6.1)] .
12.2 Pharmacodynamics
At a dose 2.3 times the maximum recommended dose, IMCIVREE does not prolong the QT interval to any clinically relevant extent.
Energy Expenditure
Short-term administration of IMCIVREE in 12 otherwise healthy patients with obesity increased resting energy expenditure and shifted substrate oxidation to fat. The safety and effectiveness of IMCIVREE have not been established in such patients and IMCIVREE is not approved to treat such patients [see Indications and Usage ( 1)].
12.3 Pharmacokinetics
The mean steady state setmelanotide C max,ss, AUC tau, and trough concentration for a 3-mg dose administered subcutaneously once daily was 31 ng/mL, 373 h*ng/mL, and 5 ng/mL, respectively simulated using individual PK model parameters from 109 adult patients with normal renal function. Steady-state plasma concentrations of setmelanotide were achieved within 2 days with daily dosing of 1-3 mg setmelanotide. The accumulation of setmelanotide in the systemic circulation during once-daily dosing over 12 weeks was approximately 30%. Setmelanotide AUC and C maxincreased proportionally following multiple-dose subcutaneous administration in the proposed dose range (1-3 mg).
Absorption
After subcutaneous injection of IMCIVREE, plasma concentrations of setmelanotide reached maximum concentrations at a median t maxof 8 h after dosing.
Distribution
The mean apparent volume of distribution of setmelanotide after subcutaneous administration of IMCIVREE 3 mg once daily was estimated to be 75.2 L. Protein binding of setmelanotide is 79.1%.
Elimination
The effective elimination half-life (t ½) of setmelanotide was approximately 11 hours. The total apparent steady state clearance of setmelanotide following subcutaneous administration of IMCIVREE 3 mg once daily was estimated to be 7.15 L/h in a typical male patient weighing 120 kg (actual body weight) with normal renal function.
Metabolism
Setmelanotide is expected to be metabolized into small peptides by catabolic pathways.
Excretion
Approximately 39% of the administered setmelanotide dose was excreted unchanged in urine during the 24-hour dosing interval following subcutaneous administration of 3 mg once daily.
Specific Populations
No clinically significant differences in the pharmacokinetics of setmelanotide were observed based on sex or disease. The effect of age 65 years or older, pregnancy, or hepatic impairment on the pharmacokinetics of setmelanotide is unknown.
Pediatric Patients
IMCIVREE has been evaluated in pediatric patients aged 2 to less than 6 years, 6 to less than 12 years, and aged 12 to 17 years. Simulations were performed using population pharmacokinetic analysis for pediatric patients aged 2 to less than 6 years, following the maximum recommended doses in each of the body weight groups - 2 mg, 1.5 mg, 1 mg, and 0.5 mg in patients weighing ≥40 kg, 30 to <40 kg, 20 to <30 kg, and 15 to <20 kg, respectively. The analyses suggest that AUC and C maxin pediatric patients aged 2 to less than 6 years are 52% and 75% higher in patients weighing ≥40 kg, 45% and 63% higher in patients weighing 30 to <40 kg, 24% and 38% higher in patients weighing 20 to <30 kg, and 17% and 14% lower in patients weighing 15 to <20 kg as compared to patients greater than or equal to 18 years (3 mg dose). For patients aged 6 to less than 12 years, the setmelanotide AUC and C maxwere 88% and 89% higher compared to patients greater than or equal to 18 years. For patients aged 12 to 17 years, the setmelanotide AUC and Cmax were both 26% higher as compared to patients greater than or equal to 18 years [see Dosage and Administration ( 2.2, 2.3, 2.4)] .
Patients with Renal Impairment
Exposure parameters, AUC 0-tand AUC 0-inf, were approximately 13%-15%, 34%-35%, and 86%-96% higher for patients with mild, moderate, and severe renal impairment, respectively, as compared to patients with normal renal function [see Dosage and Administration ( 2.5)].
Renal impairment did not appear to affect plasma protein binding. The average fraction unbound (f u) was approximately 0.2 and was independent of renal function.
Drug Interaction Studies
In vitro assessment of drug-drug interactions
Setmelanotide has low potential for pharmacokinetic drug-drug interactions related to cytochrome P450 (CYP), transporters and plasma protein binding.
In vivo assessment of drug-drug interactions
No clinical studies evaluating the drug-drug interaction potential of setmelanotide have been conducted.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of setmelanotide or of other setmelanotide products.
In patients with BBS or in patients with POMC, PCSK1, or LEPR deficiency , there is insufficient information to characterize the ADA response to setmelanotide and the effects of ADA on pharmacokinetics, pharmacodynamics, safety, or effectiveness of setmelanotide products.
During the 1-year treatment period in Study 3 in patients with obesity due to LEPR deficiency [see Clinical Studies ( 14.2)], 3/7 (43%) of IMCIVREE-treated patients developed antibodies to endogenous alpha-melanocyte stimulating hormone (MSH). Of these 3 patients, 2 tested positive post-IMCIVREE treatment and 1 was positive pre-treatment. Because of the limited sample size, the effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of setmelanotide products or consequences from these antibodies against endogenous alpha-MSH could not be determined. None of the IMCIVREE-treated patients with POMC-deficiency developed antibodies to alpha-MSH.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Setmelanotide was not carcinogenic in Tg.rasH2 mice at doses up to 10 mg/kg/day when given subcutaneously for 26 weeks.
Setmelanotide was not mutagenic or clastogenic in a bacterial reverse mutation test, an in vitrochromosome aberration test in human lymphocyte cultures, or an in vivobone marrow micronucleus study in rats.
There were no effects on the fertility of male rats subcutaneously administered up to 3.0 mg/kg/day setmelanotide, which represents 9 times the MRHD of 3 mg, based on AUC. No effects on the fertility of female rats were observed with subcutaneous administration up to 5 mg/kg/day setmelanotide, which represents 11 times the MRHD of 3 mg, based on AUC.
14. Clinical Studies
14.1 Bardet-Biedl Syndrome (Adults and Pediatric Patients Aged 6 Years and Older)
The safety and efficacy of IMCIVREE for weight reduction in adults and pediatric patients aged 6 years and older with obesity and a clinical diagnosis of Bardet-Biedl syndrome (BBS) were assessed in a 66-week clinical study, which included a 14 week randomized, double-blind, placebo-controlled period and a 52-week open-label period (Study 1 [NCT03746522]). The study enrolled patients aged 6 years and above with obesity and a clinical diagnosis of BBS. Adult patients had a BMI of ≥30 kg/m 2and pediatric patients had weight ≥97 thpercentile using growth chart assessments.
In Study 1, eligible patients entered a 14-week, randomized, double-blind, placebo-controlled treatment period (Period 1) in which patients received IMCIVREE or placebo, followed by a 52-week open-label treatment period (Period 2) in which all patients received IMCIVREE. To maintain the blind during Period 1, dose titration to a fixed dose of 3 mg given subcutaneously once daily was performed during the first 2 weeks of both Period 1 and Period 2.
Efficacy analyses were conducted in 44 patients at the end of Period 1 (Week 14, placebo-controlled data) and in 31 patients during the active-treatment period, defined as the period from randomization to Week 52 in patients initially randomized to IMCIVREE, and from Week 14 to Week 66 in patients initially randomized to placebo. Analyses of the active-treatment period include patients who had either completed 52 weeks from the start of IMCIVREE treatment or discontinued the study early at the time of the prespecified data cutoff.
A total of 44 patients with obesity and a clinical diagnosis of BBS were enrolled; 50% were adults, 32% were aged 12 to <18 years, and 18% were aged 6 to <12 years; 46% were male; 77% were White, 5% were Black or African American, 2% were Asian, and 16% had an unknown or not reported race; 2% were Hispanic or Latino ethnicity and 14% had an unknown or not reported ethnicity; and the mean BMI was 41.5 kg/m 2(range: 24.4-66.1 kg/m 2) at baseline.
Effect of IMCIVREE on BMI in Patients with Obesity and a Clinical Diagnosis of BBS
In patients aged ≥6 years with obesity and a clinical diagnosis of BBS in Study 1, the mean percent change in BMI after 52 weeks of IMCIVREE treatment was -7.9% ( Table 6), 61.3% of patients achieved a ≥5% BMI decrease from baseline, and 38.7% had a ≥10% decrease in BMI ( Table 7).
Table 6: Percent Change from Baseline in BMI after 52 Weeks from the Start of IMCIVREE Treatment in Patients Aged ≥6 Years with Obesity and a Clinical Diagnosis of BBS (Study 1)*
|
Abbreviations: CI = confidence interval; SD = standard deviation *BBS patients (N=31) who completed 52 weeks from the start of IMCIVREE treatment or discontinued the study early. Five patients who discontinued study early were defined as 0 percent change. |
|
| Statistic | Result |
| Baseline BMI (kg/m 2) | |
| Mean (SD) | 41.8 (9.0) |
| Median | 41.5 |
| Min, Max | 24.4, 61.3 |
| BMI after 52 Weeks (kg/m 2) | |
| Mean (SD) | 38.6 (9.2) |
| Median | 39.1 |
| Min, Max | 20.4, 60.9 |
| 95% CI | 35.2, 41.9 |
| Percent Change from Baseline to 52 Weeks (%) | |
| Mean (SD) | -7.9 (6.7) |
| Median | -8.8 |
| Min, Max | -25.4, 5.3 |
| 95% CI | -10.4, -5.5 |
|
Abbreviations: CI = confidence interval; SD = standard deviation *BBS patients (N=31) who completed 52 weeks from the start of IMCIVREE treatment or discontinued the study early. Five patients who discontinued study early were defined as not achieving 5% or 10% reduction. |
||
| Parameter | Statistic | Result |
| Patients* Achieving at Least 5% BMI Loss at 52 Weeks | % | 61.3 |
| 95% CI | 42.2, 78.2 | |
| Patients* Achieving at Least 10% BMI Loss at 52 Weeks | % | 38.7 |
| 95% CI | 21.8, 57.8 | |
During the 14-week double-blind, placebo-controlled portion of Study 1 (Period 1), there was a statistically significant difference in BMI reduction between the IMCIVREE-treated group and the placebo-treated group ( Table 8).
Table 8. Percent Change from Baseline in BMI after 14 Weeks of Treatment in Patients Aged ≥6 Years with Obesity and a Clinical Diagnosis of BBS (Study 1)*
|
Abbreviations: CI = confidence interval; SD = standard deviation *BBS subjects who completed the 14-week double-blind, placebo-controlled period (N=44). |
||
| Parameter | IMCIVREE
(N = 22) | Placebo
(N = 22) |
| Baseline BMI (SD) | 41.4 (10.0) | 41.6 (10.1) |
| BMI at 14 Weeks (SD) | 39.5 (9.9) | 41.6 (9.9) |
| Percent Change from Baseline to 14 Weeks (SD) | -4.6 (4.1) | -0.1 (2.3) |
| Placebo-Adjusted Difference | -4.5 | |
| 95% CI | -6.5, -2.5 | |
Effect of IMCIVREE on Hunger in Patients with Obesity and a Clinical Diagnosis of BBS
In Study 1, patients 12 years and older who were able to self-report their hunger (n=14), recorded their daily maximal hunger in a diary, which was then assessed by the Daily Hunger Questionnaire Item 2. Hunger was scored on an 11-point scale from 0 (“not hungry at all”) to 10 (“hungriest possible”). Weekly means of daily maximal hunger scores after 52 weeks from the start of IMCIVREE treatment are summarized in Table 9.
Hunger scores decreased in IMCIVREE-treated patients during the 14-week placebo-controlled period and during the open-label treatment period.
Table 9: Daily Hunger Scores – Change from Baseline in IMCIVREE-Treated Patients Aged ≥12 Years with Obesity and a Clinical Diagnosis of BBS After 52 Weeks From the Start of IMCIVREE Treatment (Study 1)
|
Abbreviations: BBS = Bardet-Biedl syndrome; CI=confidence interval; Max=maximum; Min=minimum; NC=Not calculated; SD=Standard Deviation. Note: Baseline is the last assessment prior to initiation of setmelanotide in both studies. Note: The Daily Hunger Questionnaire is not administered to patients <12 years or to patients with cognitive impairment as assessed by the Investigator. |
||
| Timepoint | Statistic | Result |
| Baseline | N | 14 |
| Mean (SD) | 6.99 (1.893) | |
| Median | 7.29 | |
| Min, Max | 4.0, 10.0 | |
| Week 52 | N | 14 |
| Mean (SD) | 4.87 (2.499) | |
| Median | 4.43 | |
| Min, Max | 2.0, 10.0 | |
| Change at Week 52 | N | 14 |
| Mean (SD) | -2.12 (2.051) | |
| Median | -1.69 | |
| Min, Max | -6.7, 0.0 | |
Supportive of IMCIVREE’s effect on weight loss, there were general numeric improvements in blood pressure, lipids, and waist circumference. However, because of the limited number of patients studied and the lack of a control group, the treatment effects on these parameters could not be accurately quantified.
14.2 POMC, PCSK1, and LEPR Deficiency (Adults and Pediatric Patients Aged 6 Years and Older)
The safety and efficacy of IMCIVREE for weight reduction in adults and pediatric patients 6 years of age and older with obesity due to POMC, PCSK1, or LEPR deficiency were assessed in 2 identically designed, 1-year, open-label studies, each with an 8 week, double-blind withdrawal period.
- Study 2 (NCT02896192) enrolled patients aged 6 years and above with obesity and genetically confirmed or suspected POMC or PCSK1 deficiency.
- Study 3 (NCT03287960) enrolled patients aged 6 years and above with obesity and genetically confirmed or suspected LEPR deficiency.
The studies enrolled patients with homozygous or presumed compound heterozygous pathogenic, likely pathogenic variants, or VUS for either the POMC or PCSK1genes (Study 2) or the LEPRgene (Study 3). In both studies, the local genetic testing results were centrally confirmed using Sanger sequencing. Patients with double heterozygous variants in 2 different genes were not eligible for treatment with IMCIVREE. In both studies, adult patients had a body mass index (BMI) of ≥30 kg/m 2. Weight in pediatric patients was ≥95 thpercentile using growth chart assessments.
IMCIVREE dose titration occurred over a 2- to 12-week period, followed by a 10-week, open-label treatment period with IMCIVREE. Patients who achieved at least a 5-kilogram weight loss (or at least 5% weight loss if baseline body weight was <100 kg) at the end of the open-label treatment period continued into a double-blind withdrawal period lasting 8 weeks, including 4 weeks of IMCIVREE followed by 4 weeks of placebo (investigators and patients were blinded to this sequence). Following the withdrawal sequence, patients re-initiated treatment with IMCIVREE at their therapeutic dose for up to 32 weeks.
Efficacy analyses were conducted in 21 patients (10 in Study 2 and 11 in Study 3) who had completed at least 1 year of treatment at the time of a prespecified data cutoff. Six additional patients enrolled in the studies (4 in Study 2 and 2 in Study 3) who had not yet completed 1 year of treatment at the time of the cutoff were not included in the efficacy analyses.
Of the 21 patients included in the efficacy analysis in Studies 2 and 3, 62% were adults and 38% were pediatric patients aged 16 years or younger.
- In Study 2, 50% of patients were female, 70% were White, and the median BMI was 40 kg/m 2(range: 26.6 53.3) at baseline.
- In Study 3, 73% of patients were female, 91% were White, and the median BMI was 46.6 kg/m 2(range: 35.8 64.6) at baseline.
Effect of IMCIVREE on Body Weight in Patients with Obesity due to POMC, PCSK1, or LEPR Deficiency
In Study 2, 80% of patients with obesity due to POMC or PCSK1 deficiency met the primary endpoint, achieving a ≥10% weight loss after 1 year of treatment with IMCIVREE.
In Study 3, 46% of patients with obesity due to LEPR deficiency achieved a ≥10% weight loss after 1 year of treatment with IMCIVREE ( Table 10).
|
Abbreviations: CI = confidence interval Note: The analysis set includes patients who received at least 1 dose of study drug and had at least 1 baseline assessment. 1From the Clopper-Pearson (exact) method 2Testing the null hypothesis: Proportion =5% |
|||
| Parameter | Statistic | Study 2
(POMC or PCSK1) (N=10) | Study 3
(LEPR) (N=11) |
| Patients Achieving at Least 10% Weight Loss at Year 1 | n (%) | 8 (80%) | 5 (46%) |
| 95% CI 1 | (44.4%, 97.5%) | (16.8%, 76.6%) | |
| P-value 2 | <0.0001 | 0.0002 | |
When treatment with IMCIVREE was withdrawn in the 16 patients who had lost at least 5 kg (or 5% of body weight if baseline body weight was <100 kg) during the 10 week open-label period in Studies 2 and 3, these patients gained an average of 5.5 kg in Study 2 and 5.0 kg in Study 3 over 4 weeks. Re initiation of treatment with IMCIVREE resulted in subsequent weight loss (see Figure 1).
|
Abbreviations: CI = confidence interval; SD = standard deviation Note: This analysis includes patients who received at least 1 dose of study drug, had at least 1 baseline assessment. 1ANCOVA model containing baseline body weight as a covariate 2Testing the null hypothesis: mean percent change=0 |
|||
| Parameter | Statistic | Study 2
(POMC or PCSK1) (N=10) | Study 3
(LEPR) (N=11) |
| Baseline Body Weight (kg) | Mean (SD) | 118.7 (37.5) | 133.3 (26.0) |
| Median | 115.0 | 132.3 | |
| Min, Max | 55.9, 186.7 | 89.4, 170.4 | |
| 1-Year Body Weight (kg) | Mean (SD) | 89.8 (29.4) | 119.2 (27.0) |
| Median | 84.1 | 120.3 | |
| Min, Max | 54.5, 150.5 | 81.7, 149.9 | |
| Percent Change from Baseline to 1 Year (%) | Mean (SD) | -23.1 (12.1) | -9.7 (8.8) |
| Median | -26.7 | -9.8 | |
| Min, Max | -35.6, -1.2 | -23.3, 0.1 | |
| LS Mean 1 | -23.12 | -9.65 | |
| 95% CI 1 | (-31.9, -14.4) | (-16.0, -3.3) | |
| P-value 2 | 0.0003 | 0.0074 | |
Figure 1: Mean Percent Change in Body Weight from Baseline in Patients Aged ≥6 Years with Obesity due to POMC, PCSK1, or LEPR Deficiency by Visit (Study 2 [N=9] and Study 3 [N=7])
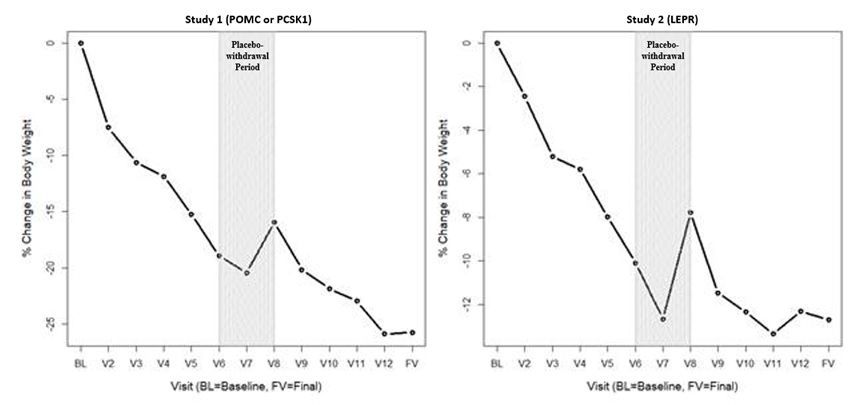
BL=Baseline (day of first dose)
V2 to V3 = variable dose titration period (2 to 12 weeks)
V3 to V6 = 10-week open-label treatment period
V6 to V8 = 8-week placebo withdrawal period (4 weeks active, 4 weeks placebo)
V8 to V12 = 32-week open-label treatment period
FV = Final visit; time point for primary efficacy analysis
Note: This figure includes patients who had lost at least 5 kg (or 5% of body weight if baseline body weight was <100 kg) during the 10-week open-label period.
Effect of IMCIVREE on Hunger in Patients with Obesity due to POMC, PCSK1, or LEPR Deficiency
In Studies 2 and 3, patients 12 years and older self-reported their daily maximal hunger in a diary, assessed by the Daily Hunger Questionnaire Item 2. Hunger was scored on an 11-point numeric rating scale from 0 (“not hungry at all”) to 10 (“hungriest possible”). Weekly means of daily hunger scores at Baseline and Week 52 are summarized in Table 12.
Table 12: Daily Hunger Scores – Change from Baseline at 1 Year in IMCIVREE-Treated Patients Aged ≥12 Years with Obesity due to POMC, PCSK1, or LEPR Deficiency in Studies 2 and 3 with Available Hunger Data
|
Note: This analysis includes patients aged 12 years and older who received at least 1 dose of study drug and had available data. Three patients in Study 2 had missing hunger data at Week 52. Hunger score was captured in a daily diary and was averaged to calculate a weekly score for analysis. Hunger ranged from 0 to 10 on an 11-point scale where 0 = “not hungry at all” and 10 = “hungriest possible.” |
|||
| Parameter | Statistic | Hunger in 24 Hours | |
| Study 2
(POMC or PCSK1(N=8) | Study 3
(LEPR) (N=8) |
||
| Baseline Hunger Score | Median | 7.9 | 7.0 |
| Median | 7.0, 9.1 | 5.0, 8.4 | |
| 1-Year Hunger Score | Min, Max | 5.5 | 4.4 |
| Min, Max | 2.5, 8.0 | 2.1, 8.0 | |
| Change from Baseline to 1 Year | Median | -2.0 | -3.4 |
| Min, Max | -6.5, -0.1 | -4.7, 1.0 | |
Hunger scores generally worsened during the double-blind, placebo withdrawal period among those patients who had experienced an improvement from baseline, and scores improved when IMCIVREE was reinitiated.
Supportive of IMCIVREE’s effect on weight loss, there were general numeric improvements in blood pressure, lipids, glycemic parameters, and waist circumference. However, because of the limited number of patients studied and the lack of a control group, the treatment effects on these parameters could not be accurately quantified.
14.3 POMC, PCSK1, and LEPR Deficiency and BBS (Pediatric Patients Aged 2 to Less Than 6 Years)
The safety and efficacy of IMCIVREE for weight reduction in pediatric patients aged 2 to less than 6 years with obesity due to POMC, PCSK1, or LEPR deficiency or BBS were assessed in a 52-week clinical trial [Study 4 (NCT04966741)]. Patients with PCSK1 deficiency were eligible but none enrolled. POMC and LEPR deficiency were confirmed by genetic testing demonstrating biallelic variants interpreted as pathogenic, likely pathogenic, or of undetermined significance; BBS was diagnosed clinically with genetic confirmation. Obesity was defined as baseline BMI ≥97th percentile for age and sex and body weight ≥20 kg.
In Study 4, IMCIVREE dose titration occurred over an 8-week period, followed by a 44-week open-label treatment period with IMCIVREE.
Twelve (12) patients were enrolled (3 patients with POMC deficiency, 4 patients with LEPR deficiency, and 5 patients with BBS); 58% were male; 58% were White, 8% were Asian, and 33% had an unknown or not reported race; 8% were Hispanic or Latino ethnicity and 17% had an unknown or not reported ethnicity; and the mean BMI was 29.9 kg/m 2(range: 19-43 kg/m 2) at baseline.
Efficacy analyses were conducted in all 12 patients at the end of treatment.
Effect of IMCIVREE on BMI in Patients Aged 2 to Less Than 6 Years with POMC or LEPR Deficiency or BBS
In Study 4, 8% of patients discontinued study drug.
The mean percent change in BMI after 52 weeks of IMCIVREE treatment was -33.8%, -13.1%, and -9.7% in patients with POMC deficiency, LEPR deficiency, and BBS, respectively ( Table 13).
|
Abbreviations: CI = confidence interval; SD = standard deviation 1Two-sided 95% CI is calculated with Student’s t-distribution. 2Using last observation carried forward (LOCF), the mean BMI (SD), median, min, and max at Week 52 are 34.9 (6.8), 32.7 29.5, and 44.9, respectively. Using observed data only, the mean BMI (SD), median, min, and max at Week 52 are 31.6 (1.9), 32.2, 29.5, and 33.1, respectively. 3Week 52 results are based off baseline observation carried forward (BOCF) for 1 patient lost to follow up after Week 8. Results using last observation carried forward are -10.8 (-34.4, 12.8) and using observed data excluding 1 patient lost to follow up are –17.4 (-37.2, 2.4). |
|||
| Statistic | POMC
(N=3) | LEPR
(N=4) | BBS
(N=5) |
| Baseline BMI (kg/m 2) | |||
| N | 3 | 4 | 5 |
| Mean (SD) | 27.8 (1.6) | 39.3 (4.8) | 23.7 (3.5) |
| Median | 28.4 | 41.2 | 23 |
| Min, Max | 26.0, 28.9 | 32.2, 42.5 | 19.3, 29.0 |
| BMI at Week 52 (kg/m 2) | |||
| N | 3 | 4 | 5 |
| Mean (SD) | 18.3 (1.2) | 34.0 (5.0) 2 | 21.4 (3.3) |
| Median | 18 | 32.7 | 22.2 |
| Min, Max | 17.3, 19.7 | 29.5, 41.1 | 17.9, 25.2 |
| Percent Change from Baseline to 52 Weeks (%) | |||
| Mean (SE) | -33.8 (4.7) | -13.1 (5.4) 3 | -9.7 (4.0) |
| Median | -37.6 | -15.1 | -9 |
| Min, Max | -39.3, -24.3 | -22.1, 0 | -21.6, 2.5 |
| 95% CI 1 | -54.1, -13.4 | -30.4, 4.2 | -20.7, 1.3 |
Supportive of IMCIVREE’s effect on weight loss, general numeric improvements in waist circumference were observed.
16. How is Imcivree supplied
IMCIVREE injection is supplied as:
- 10 mg/mL, clear to slightly opalescent, colorless to slightly yellow solution in a 1-mL multiple-dose vial for single patient use
- Package of 1 multiple-dose vial: NDC 72829-010-01
Store unopened IMCIVREE vials in the refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton. After removal from the refrigerator, vials may be kept at temperatures ranging from refrigerated to room temperature (2°C to 25°C [36°F to 77°F]) for up to 30 days with brief excursions up to 30°C (86°F). After the vial is punctured (opened), discard after 30 days. See Table 14for a summary of storage conditions for IMCIVREE. Store vials in the original carton.
Table 14 Recommended Storage for IMCIVREE Vials
|
1If necessary, IMCIVREE may be stored at room temperature (≤30°C [≤86°F]) and then returned to refrigerated conditions |
||
| Storage Condition | Unopened Vial | Opened Vial |
| 2°C to 8°C (36°F to 46°F) | Until the expiration date | Up to 30 days, OR
Until the expiration date (whichever is earlier) |
| 2°C to 25°C (36°F to 77°F) with excursions permitted up to 30°C (86°F) 1 | Up to 30 days, OR
Until the expiration date (whichever is earlier) | Up to 30 days, OR
Until the expiration date (whichever is earlier) |
| >30°C (>86°F) | Discard and do not use | Discard and do not use |
17. Patient Counseling Information
Advise the patient or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Disturbance in Sexual Arousal
Inform patients that sexual adverse reactions, including spontaneous erection, may occur in patients treated with IMCIVREE. Advise patients to seek emergency medical treatment if an erection lasts longer than 4 hours [see Warnings and Precautions ( 5.1)].
Depression and Suicidal Ideation
Inform patients or caregivers that IMCIVREE may cause depression or suicidal ideation. Advise patients or caregivers to report any new or worsening symptoms of depression, suicidal thoughts or behaviors, or unusual changes in mood or behavior [see Warnings and Precautions ( 5.2)].
Hypersensitivity Reactions
Inform patients that serious hypersensitivity reactions have been reported with use of IMCIVREE. Advise patients on the symptoms of hypersensitivity reactions and instruct them to stop taking IMCIVREE and seek medical advice promptly if such symptoms occur [see Warnings and Precautions ( 5.3)].
Skin Hyperpigmentation, Darkening of Pre-Existing Nevi, and Development of New Melanocytic Nevi
Inform patients or caregivers that skin darkening occurs in the majority of patients treated with IMCIVREE because of its mechanism of action. This change is reversable upon discontinuation of IMCIVREE. Inform patients and caregivers that the development of new melanocytic nevi may occur. Inform patients or caregivers that they should have a full body skin examination before starting and during treatment with IMCIVREE to monitor these changes [see Warnings and Precautions ( 5.4)].
Pregnancy
Advise patients who may become pregnant to inform their healthcare provider of a known or suspected pregnancy [see Use in Specific Populations ( 8.1)].
Lactation
Advise patients that treatment with IMCIVREE is not recommended while breastfeeding [see Use in Specific Populations ( 8.2)].
Administration
Instruct patients and caregivers how to prepare and administer the correct dose of IMCIVREE and assess their ability to inject subcutaneously to ensure the proper administration of IMCIVREE. Instruct patients to use a 1 mL syringe with a 28- or 29-gauge needle appropriate for subcutaneous injection. [see Dosage and Administration ( 2.6)].
Manufactured for:
Rhythm Pharmaceuticals, Inc.
222 Berkeley Street, Suite 1200
Boston, MA 02116
© 2024, Rhythm Pharmaceuticals, Inc. All rights reserved.
IMCIVREE is a registered trademark of Rhythm Pharmaceuticals, Inc.
|
PATIENT INFORMATION
IMCIVREE™ [im-SIV-ree]
|
|
|
What is IMCIVREE?
It is not known if IMCIVREE is safe and effective in children under 2 years of age. |
|
|
Do not use IMCIVREE if youhave had a serious allergic reaction to setmelanotide or any of the ingredients in IMCIVREE. Serious allergic reactions, including a severe allergic reaction called anaphylaxis, can happen when you use IMCIVREE. See the end of this Patient Information leaflet for a complete list of ingredients in IMCIVREE. |
|
|
Before using IMCIVREE, tell your healthcare provider about all your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
|
|
How should I use IMCIVREE?
|
|
|
What are the possible side effects of IMCIVREE? IMCIVREE may cause serious side effects, including:
The most common side effects of IMCIVREE include:
These are not all the possible side effects of IMCIVREE. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
|
|
|
General information about the safe and effective use of IMCIVREE. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use IMCIVREE for a condition for which it was not prescribed. Do not give IMCIVREE to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about IMCIVREE that is written for health professionals. |
|
|
What are the ingredients in IMCIVREE? Active ingredient:setmelanotide Inactive ingredients:benzyl alcohol, carboxymethylcellulose sodium, edetate disodium dihydrate, N-(carbonyl-methoxypolyethylene glycol 2000)-1, 2-distearoyl- glycero-3-phosphoethanolamine sodium salt, mannitol, phenol, hydrochloric acid, sodium hydroxide and water for injection. |
INSTRUCTIONS FOR USE
IMCIVREE™ [im-SIV-ree]
(setmelanotide)
injection, for subcutaneous use
This Instructions for Use contains information on how to inject IMCIVREE. Read and follow these instructions before injecting IMCIVREE.
Important Information You Need to Know Before Injecting IMCIVREE
- IMCIVREE is for injection under the skin only (subcutaneous injection). Do notinject IMCIVREE into a vein or muscle.
- The IMCIVREE vial may be used to give more than 1 dose of medicine (multiple-dose vial) but is for single patient use only and should not be shared with other patients.
- Inject IMCIVREE 1 time each day when you first wake up.
- Take IMCIVREE with or without food.
- IMCIVREE is given by you or a caregiver. A healthcare provider will show you or your caregiver how to inject your dose of IMCIVREE before you inject for the first time. Ask your healthcare provider or call IMCIVREE Guidance, Partnership, Support (GPS) at 1-844-YOUR-GPS (1-844-968-7477) if you have questions.
- Store opened vials of IMCIVREE in the refrigerator between 36°F to 46°F (2°C to 8°C). If needed, opened vials may be removed from the refrigerator and stored at temperatures ranging from refrigerated to room temperature (36°F to 77°F (2°C to 25°C)). Vials may be returned to the refrigerator. Throw away all opened vials 30 days after first opening, even if some medicine is still left.
- Unopened vials of IMCIVREE may be stored in the refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date. If needed, unopened vials may also be removed from the refrigerator and stored at temperatures ranging from refrigerated to room temperature (36°F to 77°F (2°C to 25°C)) for up to 30 days. Vials may be returned to the refrigerator. Throw away IMCIVREE if it has been more than 30 days since the vial was first removed from the refrigerator.
- If necessary, vials may be stored at room temperature below 86°F (30°C) and may be returned to the refrigerator. Throw away IMCIVREE that has been stored above 86°F (30°C).
- Write the date on the carton when you first open the vial.
Important note:
- Only use the syringes and needles provided to you for use with IMCIVREE.
- Always use a new syringe and needle for each injection to prevent contamination.
- Throw away used syringes and needles in a puncture-resistant, disposable sharps container as soon as you finish giving the injection. See “Disposing of IMCIVREE”at the end of these instructions.
- Do notreuse or share your needles with other people.
- Do notrecap the needle. Recapping the needle can lead to a needle stick injury.
- Keep IMCIVREE, needles, syringes, and all medicines out of the reach of children.
Understanding Your IMCIVREE Dose
Calculate the number of doses of IMCIVREE in each vial:
- Each unopened IMCIVREE vial contains 10 milligrams (mg) of medicine in 1 milliliter (mL) of solution.
- The vial will contain both medicine and air. Most of the vial will be filled with air.
- Your healthcare provider will determine your dose of medicine in milligrams (mg).
- The IMCIVREE vial may be used to give more than 1 dose of medicine (multiple-dose vial).
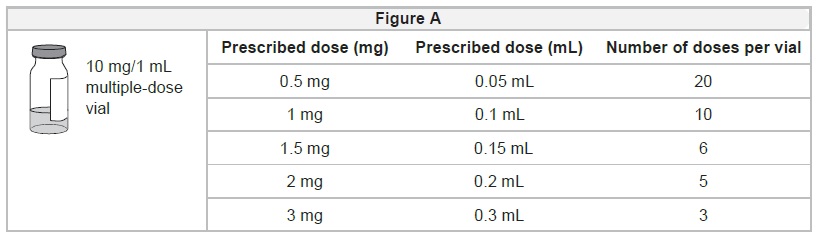
- Use Figure Ato see how many times you may use each vial based on your prescribed dose.
- Do notuse more doses from a single vial than listed in Figure A
Preparing to Inject IMCIVREE
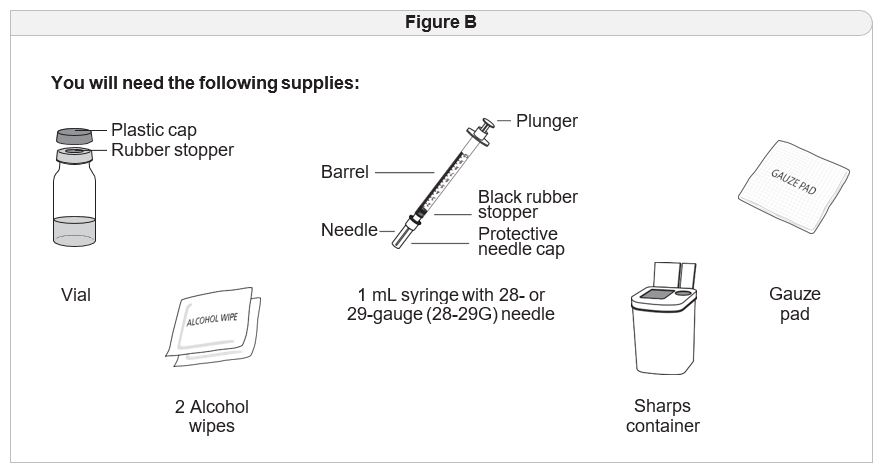
Step 1 Gather your supplies.
- Gather the supplies you will need for your injection ( Figure B).
- Place your supplies on a clean, flat work surface.
|
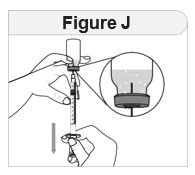
Step 2 Check your IMCIVREE vial.
|
|
|
|
|
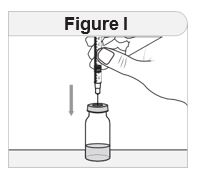
Step 3 Prepare your IMCIVREE vial.
|
|
|
|
|
|
|
|
|
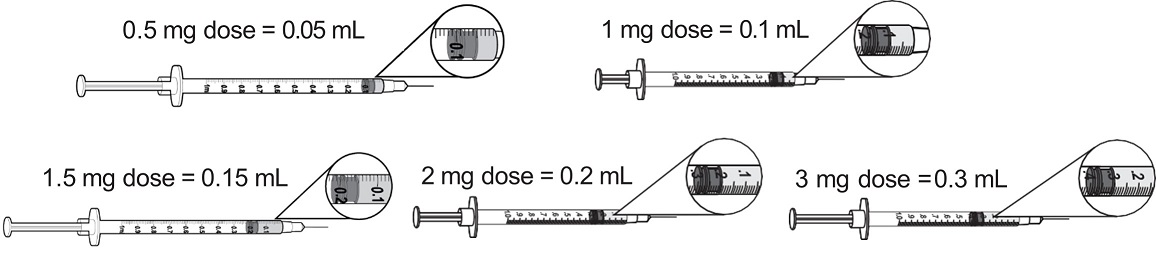
Step 4 Prepare the syringe. When measuring your dose, be sure to read the markings starting from the end closest to the black rubber stopper ( Figure G). |
|
|
|
|
|
|
|
|
|
|
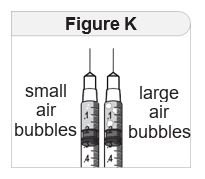
What to do if you see large air bubbles: Large air bubbles can reduce the dose of medicine you receive. To remove large air bubbles:
|
|
|
|
|
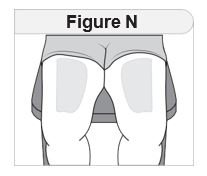
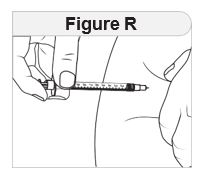
Injecting IMCIVREE Step 5 Prepare your injection site. |
|
|
|
|
|
|
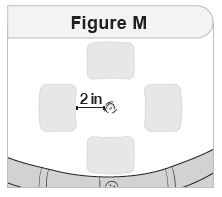
Rotate your injection site each day. You should use a different injection site each time you give an injection, at least 1 inch away from the area you used for your previous injection. You may want to use a calendar or diary to record your injection sites. |
|
|
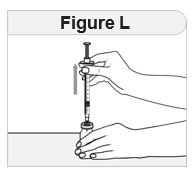
Step 6 Place your hands for the injection. |
|
|
|
|
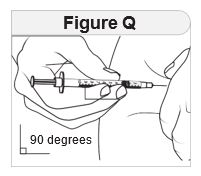
Step 7 Inject and release. |
|
|
|
|
|
|
Tips for giving injections to children When giving a child an injection, it can help to have the child do other things. Have the child:
|
|
|
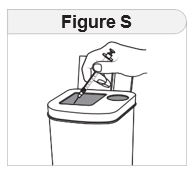
Storing IMCIVREE
|
|
|
|
|
|
|
For more information about IMCIVREE, including how to inject IMCIVREE, go to www.IMCIVREE.comor call 1-844-YOUR-GPS (1-844-968-7477).
IMCIVREE is Manufactured for:
Rhythm Pharmaceuticals, Inc
222 Berkeley Street, Suite 1200
Boston, MA 02116
Made in France
IMCIVREE is a trademark of Rhythm Pharmaceuticals, Inc.
©2020 Rhythm Pharmaceuticals, Inc.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Revised [March 2025]
| IMCIVREE
setmelanotide solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Rhythm Pharmaceuticals, Inc (061479535) |
More about Imcivree (setmelanotide)
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: melanocortin receptor agonists
- En español