Fem pH Vaginal Jelly: Package Insert / Prescribing Info
Package insert / product label
Generic name: acetic acid and oxyquinoline sulfate
Dosage form: vaginal jelly
Drug class: Topical anti-infectives
Medically reviewed by Drugs.com. Last updated on Jan 13, 2025.
On This Page
Fem pH Vaginal Jelly Description
Fem pH Vaginal Jelly is a bland, non-irritating water dispersible, buffered acid jelly for intravaginal use. Fem pH is classified as a Vaginal Therapeutic Jelly. Fem pH contains 0.9% glacial acetic acid (C2H402) and 0.025% oxyquinoline sulfate (C18H16N206S) compounded with glycerin, lactic acid, poly ethylene glycol 4500 and purified water. Fem pH is formulated to pH 3.8-4.3 and is adjusted using 1 N potassium hydroxide.
Fem pH Vaginal Jelly - Clinical Pharmacology
Fem pH acts to restore and maintain normal vaginal acidity through its buffered action.
Indications and Usage for Fem pH Vaginal Jelly
Fem pH is indicated as adjunctive therapy in those cases where restoration and maintenance of vaginal acidity is desirable.
Warnings
No serious adverse reactions or potential safety hazard have been reported with the use of Fem pH.
Precautions
Drug Interactions
No incidence of drug interactions has been reported with concomitant use of Fem pH and any other medication.
Laboratory Tests
The monitoring of vaginal acidity (pH) may be helpful in following the patient's response. (The normal vaginal pH has been shown to be in the range of 4.0 to 5.0)
Carcinogenesis
No long-term studies in animals have been performed to evaluate carcinogenic potential.
Related/similar drugs
Fem pH Vaginal Jelly Dosage and Administration
The usual dose is one applicator full, administered intra-vaginally, morning and evening. Duration of treatment may be determined by the patient's response to therapy. Each tube has a tamper evident seal at the opening of the tube. Replace cap after each use. To fill applicator screw applicator clockwise onto the tube. Squeeze tube forcing Fem pH jelly into barrel until it is full. Then unscrew applicator counter-clockwise to remove from tube. Lie on your back with knees drawn up. Hold filled applicator by the barrel and gently insert it into the vagina as far as it will comfortably go. Press plunger to empty the contents. Keep the plunger depressed and remove the applicator from vagina. After each use pull applicator apart and wash with warm soapy water, rinse well, dry and reassemble.
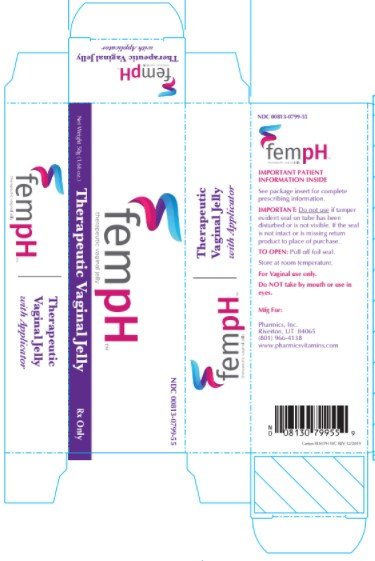
Therapeutic Vaginal Jelly
Directions for using Fem pH applicator.
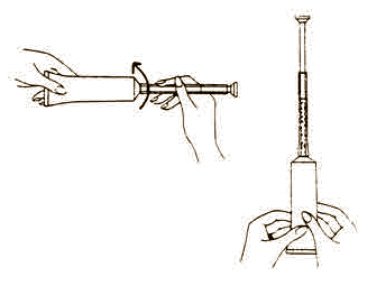
Each tube has a tamper evident seal at the opening of the tube. Replace cap after each use.
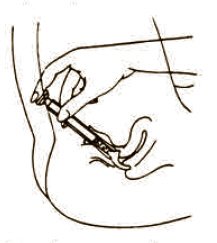 |
To fill applicator screw applicator clockwise onto the tube. Squeeze tube forcing Fem pH jelly into barrel until it is full. Then unscrew applicator counter-clockwise to remove from tube. Lie on your back with knees drawn up. Hold filled applicator by the barrel and gently insert it into the vagina as far as it will comfortably go. Press plunger to empty the contents. Keep the plunger depressed and remove the applicator from vagina. |
After each use pull applicator apart and wash with warm soapy water, rinse well, dry and reassemble.

| FEM PH
acetic acid and oxyquinoline sulfate jelly |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Pharmics, Inc. (058560996) |
| Registrant - Womens Choice (833067841) |