8-MOP: Package Insert / Prescribing Info
Package insert / product label
Generic name: methoxsalen
Dosage form: capsule, gelatin coated
Drug class: Psoralens
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
The 8-MOP brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
On This Page
Rx only
CAUTION: METHOXSALEN IS A POTENT DRUG. READ ENTIRE BROCHURE PRIOR TO PRESCRIBING OR DISPENSING THIS MEDICATION.
Methoxsalen with UV radiation should be used only by physicians who have special competence in the diagnosis and treatment of psoriasis and vitiligo and who have special training and experience in photochemotherapy. Psoralen and ultraviolet radiation therapy should be under constant supervision of such a physician. For the treatment of patients with psoriasis, photochemotherapy should be restricted to patients with severe, recalcitrant, disabling psoriasis which is not adequately responsive to other forms of therapy, and only when the diagnosis is certain. Because of the possibilities of ocular damage, aging of the skin, and skin cancer (including melanoma), the patient should be fully informed by the physician of the risks inherent in this therapy. When methoxsalen is used in combination with photopheresis, refer to the UVAR* System Operator's Manual for specific warnings, cautions, indications, and instructions related to photopheresis.
CAUTION: 8-MOP® Capsules (Methoxsalen Hard Gelatin Capsules) may not be interchanged with Oxsoralen-Ultra® Capsules (Methoxsalen Soft Gelatin Capsules) without retitration of the patient.
8-MOP Description
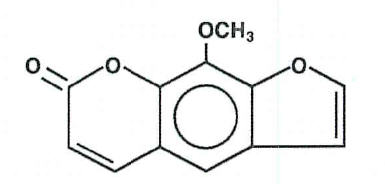
8-MOP (Methoxsalen, 8-Methoxypsoralen) Capsules, 10mg. Methoxsalen is a naturally occurring photoactive substance found in the seeds of the Ammi majus (Umbelliferae) plant and in the roots of Heracleum Candicans. It belongs to a group of compounds known as psoralens, or furocoumarins. The chemical name of methoxsalen is 9-methoxy-7 H-furo[3,2-g][1]-benzopyran-7-one; it has the following structure:
8-MOP - Clinical Pharmacology
The combination treatment regimen of psoralen (P) and ultraviolet radiation of 320-400 nm wavelength commonly referred to as UVA is known by the acronym, PUVA. Skin reactivity to UVA (320-400 nm) radiation is markedly enhanced by the ingestion of methoxsalen. The drug reaches its maximum bioavailability 1 1/2-3 hours after oral administration and may last for up to 8 hours (Pathak et al., 1974)1. Methoxsalen is reversibly bound to serum albumin and is also preferentially taken up by epidermal cells (Artuc et al. 1979)2. At a dose which is six times larger than that used in humans, it induces mixed function oxidases in the liver of mice (Mandula et al. 1978)3. In both mice and man, methoxsalen is rapidly metabolized. Approximately 95% of the drug is excreted as a series of metabolites in the urine within 24 hours (Pathak et al. 1977)4.
The exact mechanism of action of methoxsalen with the epidermal melanocyctes and keratinocytes is not known. The best known biochemical reaction of methoxsalen is with DNA. Methoxsalen, upon photoactivation, conjugates and forms covalent bonds with DNA which leads to the formation of both monofunctional (addition to a single strand of DNA) and bifunctional adducts (crosslinking of psoralen to both strands of DNA) (Dall' Acqua et at., 19715; Cole, 19706; Musajo et al., 19747; Dall' Acqua et al., 19798). Reactions with proteins have also been described (Yoshikawa, et al., 19799).
Methoxsalen acts as a photosensitizer. Administration of the drug and subsequent exposure to UVA can lead to cell injury. Orally administered methoxsalen reaches the skin via the blood and UVA penetrates well into the skin. If sufficient cell injury occurs in the skin, an inflammatory reaction occurs. The most obvious manifestation of this reaction is delayed erythema, which may not begin for several hours and peaks at 48-72 hours. The inflammation is followed, over several days to weeks, by repair which is manifested by increased melanization of the epidermis and thickening of the stratum corneum. The mechanisms of therapy are not known. In the treatment of vitiligo, it has been suggested that melanocytes in the hair follicle are stimulated to move up the follicle and to repopulate the epidermis (Ortonne et al. 197910). In the treatment of psoriasis, the mechanism is most often assumed to be DNA photodamage and resulting decrease in cell proliferation but other vascular, leukocyte, or cell regulatory mechanisms may also be playing some role. Psoriasis is a hyperproliferative disorder and other agents known to be therapeutic for psoriasis are known to inhibit DNA synthesis.
Indications and Usage for 8-MOP
A. Photochemotherapy (methoxsalen with long wave UVA radiation) is indicated for the symptomatic control of severe, recalcitrant, disabling psoriasis not adequately responsive to other forms of therapy and when the diagnosis has been supported by biopsy. Photochemotherapy is intended to be administered only in conjunction with a schedule of controlled doses of long wave ultraviolet radiation.
B. Photochemotherapy (methoxsalen with long wave ultraviolet radiation) is indicated for the repigmentation of idiopathic vitiligo.
C. Photopheresis (methoxsalen with long wave ultraviolet radiation of white blood cells) is indicated for use with the UVAR* System in the palliative treatment of the skin manifestations of cutaneous T-cell lymphoma (CTCL) in persons who have not been responsive to other forms of treatment. While this dosage form of methoxsalen has been approved for use in combination with photopheresis. Oxsoralen Ultra® Capsules have not been approved for that use.
Contraindications
A. Patients exhibiting idiosyncratic reactions to psoralen compounds.
B. Patients possessing a specific history of light sensitive disease states should not initiate methoxsalen therapy. Diseases associated with photosensitivity include lupus erythematosus, porphyria cutanea tarda, erythropoietic protoporphyria, variegate porphyria, xeroderma pigmentosum, and albinism.
C. Patients exhibiting melanoma or possessing a history of melanoma.
D. Patients exhibiting invasive squamous cell carcinomas.
E. Patients with aphakia, because of the significantly increased risk of retinal damage due to the absence of lenses.
Warnings
A. SKIN BURNING
Serious burns from either UVA or sunlight (even through window glass) can result if the recommended dosage of the drug and/or exposure schedules are not maintained.
B. CARCINOGENICITY
1. ANIMAL STUDIES
Topical or intraperitoneal methoxsalen has been reported to be a potent photocarcinogen in albino mice and hairless mice. However, methoxsalen given by the oral route to albino mice or by any route in pigmented mice is considerably less phototoxic or carcinogenic (Hakim et at. 196011; Pathak et al. 195912).
2. HUMAN STUDIES
A prospective study of 1380 patients over 5 years revealed an approximately nine-fold increase in risks of squamous cell carcinoma among PUVA treated patients (Stern et al. 197913 and Stern et al. 198014). This increase in risk appears greatest among patients who are fair skinned or had pre-PUVA exposure to 1) prolonged tar and UVB treatment, 2) ionizing radiation, or 3) arsenic.
In addition, an approximately two-fold increase in the risk of basal cell carcinoma was noted in this study. Roenigk et al. 198015 studied 690 patients for up to 4 years and found no increase in the risk of non-melanoma skin cancer. However, patients in this cohort had significantly less exposure to PUVA than in the Stern et al study. Recent analysis of new data in the Stern et al cohort (Stern et al., 199716) has shown that these patients had an elevated relative risk of contracting melanoma. The relative risk for melanoma in these patients was 2.3 (95 percent confidence interval 1.1 to 4.1). The risk is particularly higher in those patients who have received more than 250 PUVA treatments and in those whose treatment has spanned greater than 15 years earlier. Some patients developing melanoma did so even after having ceased PUVA therapy over 5 years earlier. These observations indicate the need for monitoring of PUVA patients for skin tumors throughout their lives.
In a study in Indian patients treated for 4 years for vitiligo, 12 percent developed keratoses, but not cancer, in the depigmented, vitiliginous areas (Mosher, 198017). Clinically, the keratoses were keratotic papules, actinic keratosis-like macules, nonscaling dome-shaped papules, and lichenoid porokeratotic-like papules.
C. CATARACTOGENICITY
1. ANIMAL STUDIES
Exposure to large doses of UVA causes cataracts in animals, and this effect is enhanced by the administration of methoxsalen (Cloud et al. 196018; Cloud et al. 196119; Freeman et al. 196920).
2. HUMAN STUDIES
It has been found that the concentration of methoxsalen in the lens is proportional to the serum level. If the lens is exposed to UVA during the time methoxsalen is present in the lens, photochemical action may lead to irreversible binding of methoxsalen to proteins and the DNA components of the lens (Lerman et al. 198021). However, if the lens is shielded from UVA, the methoxsalen will diffuse out of the lens in a 24 hour period21. Patients should be told emphatically to wear UVA-absorbing, wraparound sunglasses for the twenty-four (24) hour period following ingestion of methoxsalen, whether exposed to direct or indirect sunlight in the open or through a window glass.
Among patients using proper eye protection, there is no evidence for a significantly increased risk of cataracts in association with PUVA therapy.13 Thirty-five of 1380 patients have developed cataracts in the five years since their first PUVA treatment. This incidence is comparable to that expected in a population of this size and age distribution. No relationship between PUVA dose and cataract risk in this group has been noted.
D. ACTINIC DEGENERATION
Exposure to sunlight and/or ultraviolet radiation may result in "premature aging" of the skin.
E. BASAL CELL CARCINOMAS
Patients exhibiting multiple basal cell carcinomas or having a history of basal cell carcinomas should be diligently observed and treated.
F. RADIATION THERAPY
Patients having a history of previous x-ray therapy or grenz ray therapy should be diligently observed for signs of carcinoma.
G. ARSENIC THERAPY
Patients having a history of previous arsenic therapy should be diligently observed for signs of carcinoma.
H. HEPATIC DISEASES
Patients with hepatic insufficiency should be treated with caution since hepatic biotransformation is necessary for drug urinary excretion.
I. CARDIAC DISEASES
Patients with cardiac diseases or others who may be unable to tolerate prolonged standing or exposure to heat stress should not be treated in a vertical UVA chamber.
J. TOTAL DOSAGE
The total cumulative dose of UVA that can be given over long periods of time with safety has not as yet been established.
K. CONCOMITANT THERAPY
Special care should be exercised in treating patients who are receiving concomitant therapy (either topically or systemically) with known photosensitizing agents such as anthralin, coal tar or coal tar derivatives, griseofulvin, phenothiazines, nalidixic acid, fluoroquinolone antibiotics, halogenated salicylanilides (bacteriostatic soaps), sulfonamides, tetracyclines, thiazides, and certain organic staining dyes such as methylene blue, toluidine blue, rose bengal, and mythyl orange.
Precautions
A. GENERAL - APPLICABLE TO BOTH VITILIGO AND PSORIASIS TREATMENT
1. BEFORE METHOXSALEN INGESTION
Patients must not sunbathe during the 24 hours prior to methoxsalen ingestion and UV exposure. The presence of a sunburn may prevent an accurate evaluation of the patient's response to photochemotherapy.
2. AFTER METHOXSALEN INGESTION
a. UVA-absorbing wrap-around sunglasses should be worn during daylight for 24 hours after methoxsalen ingestion. The protective eyewear must be designed to prevent entry of stray radiation to the eyes, including that which may enter from the sides of the eyewear. The protective eyewear is used to prevent the irreversible binding of methoxsalen to the proteins and DNA components of the lens. Cataracts form when enough of the binding occurs. Visual discrimination should be permitted by the eyewear for patient well-being and comfort.
b. Patients must avoid sun exposure, even through window glass or cloud cover, for at least 8 hours after methoxsalen ingestion. If sun exposure cannot be avoided, the patient should wear protective devices such as a hat and gloves, and/or apply sunscreens which contain ingredients that filter out UVA radiation (e.g., sunscreens containing benzophenone and/or PABA esters which exhibit a sun protective factor equal to or greater than 15). These chemical sunscreens should be applied to all areas that might be exposed to the sun (including lips). Sunscreens should not be applied to areas affected by psoriasis until after the patient has been treated in the UVA chamber.
3. DURING PUVA THERAPY
a. Total UVA-absorbing/blocking goggles mechanically designed to give maximal ocular protection must be worn. Failure to do so may increase the risk of cataract formation. A reliable radiometer can be used to verify elimination of UVA transmission through the goggles.
b. Abdominal skin, breasts, genitalia, and other sensitive areas should be protected for approximately 1/3 of the initial exposure time until tanning occurs.
c. Unless affected by disease, male genitalia should be shielded.
4. AFTER COMBINED METHOXSALEN/UVA THERAPY
a. UVA-absorbing wrap-around sunglasses should be worn during the daylight for 24 hours after combined methoxsalen/UVA therapy.
b. Patients should not sunbathe for 48 hours after therapy. Erythema and/or burning due to photochemotherapy and sunburn due to sun exposure are additive.
5. VITILIGO THERAPY
a. The dosage of methoxsalen should not be increased above 0.6 mg/kg since overdosage may result in serious burning of the skin.
b. Eye and skin sun protection as described in the Precautions - General section should be observed.
C. LABORATORY TESTS
1. Patients should have an ophthalmologic examination prior to the start of therapy, and thence yearly.
2. Patients should have the following tests prior to the start of therapy and should be retested 6-12 months subsequently. Additional tests at more extended time periods should be conducted as clinically indicated.
a. Complete Blood Count (Hemoglobin or Hematocrit; White Blood Count - if abnormal, a differential count).
b. Anti-nuclear Antibodies.
c. Liver Function Tests.
d. Renal Function Tests (Creatinine or Blood Urea Nitrogen).
G. NURSING MOTHERS
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when methoxsalen is administered to a nursing woman.
H. PEDIATRIC USE
Safety in children has not been established. Potential hazards of long-term therapy include the possibilities of carcinogenicity and cataractogenicity as described in the Warnings Section as well as the probability of actinic degeneration which is also described in the Warnings Section.
Adverse Reactions/Side Effects
A. METHOXSALEN
The most commonly reported side effect of methoxsalen alone is nausea, which occurs with approximately 10% of all patients. This effect may be minimized or avoided by instructing the patient to take methoxsalen with milk or food, or to divide the dose into two portions, taken approximately one-half hour apart. Other effects include nervousness, insomnia, and psychological depression.
B. COMBINED METHOXSALEN/UVA THERAPY
1. PRURITUS
This adverse reaction occurs with approximately 10% of all patients. In most cases, pruritus can be alleviated with frequent application of bland emollients or other topical agents; severe pruritus may require systemic treatment. If pruritus is unresponsive to these measures, shield pruritic areas from further UVA exposure until the condition resolves. If intractable pruritus is generalized, UVA treatment should be discontinued until the pruritus disappears.
2. ERYTHEMA
Mild, transient erythema at 24-48 hours after PUVA therapy is an expected reaction and indicates that a therapeutic interaction between methoxsalen and UVA occurred. An area showing moderate erythema (greater than Grade 2 – See Table 1 for grades of erythema) should be shielded during subsequent UVA exposures until the erythema has resolved. Erythema greater than Grade 2 which appears within 24 hours after UVA treatment may signal a potentially severe burn. Erythema may become progressively worse over the next 24 hours, since the peak erythemal reaction characteristically occurs 48 hours or later after methoxsalen ingestion. The patient should be protected from further UVA exposures and sunlight, and should be monitored closely.
3. IMPORTANT DIFFERENCES BETWEEN PUVA ERYTHEMA AND SUNBURN
PUVA-induced inflammation differs from sunburn or UVB phototherapy in several ways. The in situ depth of photochemistry is deeper within the tissue because UVA is transmitted further into the skin. The DNA lesions induced by PUVA are very different from UV-induced thymine dimers and may lead to a DNA crosslink. This DNA lesion may be more problematic to the cell because crosslinks are more lethal and psoralen-DNA photoproducts may be "new" or unfamiliar substrates for DNA repair enzymes. DNA synthesis is also suppressed longer after PUVA. The time course of delayed erythema is different with PUVA and may not involve the usual mediators seen in sunburn. PUVA-induced redness may be just beginning at 24 hours, when UVB erythema has already passed its peak. The erythema dose-response curve is also steeper for PUVA. Compared to equally erythemogenic doses of UVB, the histologic alterations induced by PUVA show more dermal vessel damage and longer duration of epidermal and dermal abnormalities.
4. OTHER ADVERSE REACTIONS
Those reported include edema, dizziness, headache, malaise, depression, hypopigmentation, vesiculation and bullae formation, non-specific rash, herpes simplex, miliaria, urticaria, folliculitis, gastrointestinal disturbances, cutaneous tenderness, leg cramps, hypotension, and extension of psoriasis.
Related/similar drugs
Overdosage
In the event of methoxsalen overdosage, induce emesis and keep the patient in a darkened room for at least 24 hours. Emesis is beneficial only within the first 2 to 3 hours after ingestion of methoxsalen, since maximum blood levels are reached by this time.
8-MOP Dosage and Administration
A. VITILIGO THERAPY
1. DRUG DOSAGE
Two capsules (10 mg each) in one dose taken with milk or in food two to four hours before ultraviolet light exposure.
B. PSORIASIS THERAPY
1. DRUG DOSAGE - INITIAL THERAPY
The methoxsalen capsules should be taken 2 hours before UVA exposure with some food or milk according to the following table:
| Patient's Weight | Dose | |
|---|---|---|
| (kg) | (lbs) | (mg) |
|
<30 |
<66 |
10 |
|
30-50 |
66-110 |
20 |
|
51-65 |
112-143 |
30 |
|
66-80 |
146-176 |
40 |
|
81-90 |
179-198 |
50 |
|
91-115 |
201-254 |
60 |
|
>115 |
>254 |
70 |
Additional drug dosage directions are as follows:
a. Weight Change: In the event that the weight of a patient changes during treatment such that he/she falls into an adjacent weight range/dose category, no change in the dose of methoxsalen is usually required. If, in the physician's opinion, however, a weight change is sufficiently great to modify the drug dose, then an adjustment in the time of exposure to UVA should be made.
b. Dose/Week: The number of doses per week of methoxsalen capsules will be determined by the patient's schedule of UVA exposures. In no case should treatments be given more often than once every other day because the full extent of phototoxic reactions may not be evident until 48 hours after each exposure.
c. Dosage Increase: Dosage may be increased by 10 mg. after the fifteenth treatment under the conditions outlined in section XI.B.4.b.
X. UVA RADIATION SOURCE SPECIFICATIONS & INFORMATION
A. IRRADIANCE UNIFORMITY
(For photopheresis, refer to the UVAR* System Operator's Manual.)
The following specifications should be met with the window of the detector held in a vertical plane:
1. Vertical variation: For readings taken at any point along the vertical center axis of the chamber (to within 15 cm from the top and bottom), the lowest reading should not be less than 70 percent of the highest reading.
2. Horizontal variation: Throughout any specific horizontal plane, the lowest reading must be at least 80 percent of the highest reading, excluding the peripheral 3 cm of the patient treatment space.
B. PATIENT SAFETY FEATURES
The following safety features should be present: (1) Protection from electrical hazard: All units should be grounded and conform to applicable electrical codes. The patient or operator should not be able to touch any live electrical parts. There should be ground fault protection. (2) Protective shielding of lamps: The patient should not be able to come in contact with the bare lamps. In the event of lamp breakage, the patient should not be exposed to broken lamp components. (3) Hand rails and hand holds: Appropriate supports should be available to the patient. (4) Patient viewing window: A window which blocks UV should be provided for viewing the patient during treatment. (5) Door and latches: Patients should be able to open the door from the inside with only slight pressure to the door. (6) Non-skid floor: The floor should be of a non-skid nature. (7) Thermoregulation: Sufficient air flow should be provided for patient safety and comfort, limiting temperature within the UVA radiator cabinet to approximately less than 100° F. (8) Timer: The irradiator should be equipped with an automatic timer which terminates the exposure at the conclusion of a pre-set time interval. (9) Patient alarm device: An alarm device within the UVA irradiator chamber should be accessible to the patient for emergency activation. (10) Danger label: The unit should have a label prominently displayed which reads as follows:
DANGER – Ultraviolet Radiation – Follow your physician's instructions – Failure to use protective eyewear may result in eye injury.
C. UVA EXPOSURE DOSIMETRY MEASUREMENTS
The maximum radiant exposure or irradiance (within ±15 percent) of UVA (320-400 nm) delivered to the patient should be determined by using an appropriate radiometer calibrated to be read in Joules/cm2 or mW/cm2. In the absence of a standard measuring technique approved by the National Bureau of Standards, the system should use a detector corrected to a cosine spatial response. The use and recalibration frequency of such a radiometer for a specific UVA irradiator chamber should be specified by the manufacturer because the UVA dose (exposure) is determined by the design of the irradiator, the number of lamps, and the age of the lamps. If irradiance is measured, the radiometer reading in mW/cm2 is used to calculate the exposure time in minutes to deliver the required UVA dose in Joules/cm2 to a patient in the UVA irradiator cabinet. The equation is:
|
Exposure Time |
= |
Desired UVA Dose (J/cm2) |
|
in minutes |
0.06 × Irradiance (mW/cm2) |
Overexposure due to human error should be minimized by using an accurate automatic timing device, which is set by the operator and controlled by energizing and de-energizing the UVA irradiator lamp. The timing device calibration interval should be specified by the manufacturer. Safety systems should be included to minimize the possibility of delivering a UVA exposure which exceeds the prescribed dose, in the event the timer or radiometer should malfunction.
XI. PUVA TREATMENT PROTOCOL
A. INITIAL EXPOSURE
The initial dosage and UVA exposure should be determined according to the guidelines presented previously under IX.B.1, and the information presented in this section.
| Skip Type | History | Recommended Joules/cm2 |
|---|---|---|
|
||
|
I |
Always burn, never tan (Patients with Erythrodermic psoriasis are to be classed as Type I for determination of UVA dosage.) |
0.5 J/cm2 |
|
II |
Always burn, but sometimes tan |
1.0 J/cm2 |
|
III |
Sometimes burn, but always tan |
1.5 J/cm2 |
|
IV |
Never burn, always tan |
2.0 J/cm2 |
|
Physician Examination | ||
|
V* |
Moderately pigmented |
2.5 J/cm2 |
|
VI* |
Blacks |
3.0 J/cm2 |
B. CLEARING PHASE
Specific recommendations for patient treatment are as follows:
1. SKIN TYPES I, II & III
Patients with skin types I, II and III may be treated 2 or 3 times per week. UVA exposure may be held constant or increased by up to 1.0 Joule/cm2 at each treatment, according to the patient's response. If erythema occurs, however, do not increase exposure time until erythema resolves. The severity and extent of the patient's erythema may be used to determine whether the next exposure should be shortened, omitted, or maintained at the previous dosage. See Adverse Reactions section for additional information.
2. SKIN TYPES IV, V & VI
Patients with skin types IV, V and VI may be treated 2 or 3 times per week. UVA exposure may be held constant or increased by up to 1.5 Joules/cm2 at each treatment unless erythema occurs. If erythema occurs, follow instructions outlined above in the procedures for patients with skin types I, II and III.
3. ERYTHRODERMIC PSORIASIS
Patients with erythrodermic psoriasis should be treated with special attention because pre-existing erythema may obscure observations of possible treatment-related phototoxic erythema. These patients may be treated 2 or 3 times per week, as a Type I patient.
4. MISCELLANEOUS SITUATIONS
a. If there is no response after a total of 10 treatments, the exposure of UVA energy may be increased by an additional 0.5-1.0 Joules/cm2 above the prior incremental increases for each treatment. (Example: a patient whose exposure dosage is being increased by 1.0 Joule/cm2 may now have all subsequent doses increased by 1.5-2.0 Joules/cm2.)
b. If there is no response, or only minimal response, after 15 treatments, the dosage of methoxsalen may be increased by 10 mg. (a one-time increase in dosage). This increased dosage may be continued for the remainder of the course of treatment but should not be exceeded.
c. If a patient misses a treatment, the UVA exposure time of the next treatment should not be increased. If more than one treatment is missed, reduce the exposure by 0.5 Joules/cm2 for each treatment missed.
d. If the lower extremities are not responding as well as the rest of the body and do not show erythema, cover all other body area and give 25 percent of the present exposure dose as an additional exposure to the lower extremities. This additional exposure to the lower extremities should be terminated if erythema develops on these areas.
e. Non-responsive psoriasis: If a patient's generalized psoriasis is not responding, or if the condition appears to be worsening during treatment, the possibility of a generalized phototoxic reaction should be considered. This may be confirmed by the improvement of the condition following temporary discontinuance of this therapy for two weeks. If no improvement occurs during the interruption of treatment, this patient may be considered a treatment failure.
C. ALTERNATIVE EXPOSURE SCHEDULE
As an alternative to increasing the UVA exposure at each treatment, the following schedule may be followed; this schedule may reduce the total number of Joules/cm2 received by the patient over the entire course of therapy.
1. Incremental increases in UVA exposure for all patients may range from 0.5 to 1.5 Joules/cm2, according to the patient's response to therapy.
2. Once Grade 2 clearing (see Table 2) has been reached and the patient is progressing adequately, UVA dosage is held constant. This dosage is maintained until Grade 4 clearing is reached.
3. If the rate of clearing significantly decreases, exposure dosage may be increased at each treatment (0.1-1.5 Joules/cm2) until Grade 3 clearing and a satisfactory progress rate is attained. The UVA exposure will be held constant again until Grade 4 clearing is attained. These increases may be used also if the rate of clearing significantly decreases between Grade 3 and Grade 4 response. However, the possibility of a phototoxic reaction should be considered; see Non-responsive Psoriasis, above.
4. In summary, this schedule raises slightly the increments (Joules/cm2) of UVA dosage, but limits these increases to those periods when the patient is not responding adequately. Otherwise, the UVA exposure is held at the lowest effective dose.
D. MAINTENANCE PHASE
The goal of maintenance treatment is to keep the patient as symptom-free as possible with the least amount of UVA exposure.
1. SCHEDULE OF EXPOSURES
When patients have achieved 95 percent clearing, or Grade 4 response (Table 2), they may be placed on the following maintenance schedules (M1 - M4), in sequence. It is recommended that each maintenance schedule be adhered to for at least 2 treatments (unless erythema or psoriatic flare occurs, in which case see (2a) and (2b) below).
| Maintenance Schedules |
|---|
|
M1 – once/week |
|
M2 – once/2 weeks |
|
M3 – once/3 weeks |
|
M4 – p.r.n. (i.e., for flares) |
2. LENGTH OF EXPOSURE
The UVA exposure for the first maintenance treatment of any schedule (except M4 as noted below) is the same as that of the patient's last treatment under the previous schedule. For skin types I-IV, however, it is recommended that the maximum UVA dosage during maintenance treatments not exceed the following:
| Skin Types | Joules/cm2/treatment |
|---|---|
|
I |
12 |
|
II |
14 |
|
III |
18 |
|
IV |
22 |
If the patient develops erythema or new lesions of psoriasis, proceed as follows:
a. Erythema: During maintenance therapy, the patient's tan and threshold dose for erythema may gradually decrease. If maintenance treatments produce significant erythema, the exposure to UVA should be decreased by 25 percent until further treatments no longer produce erythema.
b. Psoriasis: If the patient develops new areas of psoriasis during maintenance therapy (but still is classified as having a Grade 4 response), the exposure to UVA may be increased by 0.5-1.5 Joules/cm2 at each treatment; this is appropriate for all types of patients. These increases are continued until the psoriasis is brought under control and the patient is again clear. The exposure being administered when this clearing is reached should be used for further maintenance treatment.
3. FLARES DURING MAINTENANCE
If the patient flares during maintenance treatment (i.e., develops psoriasis on more than 5 percent of the originally involved areas of the body) his maintenance treatment schedule may be changed to the preceding maintenance or clearing schedule. The patient may be kept on his schedule until again 95 percent clear. If the original maintenance treatment schedule is unable to control the psoriasis, the schedule may be changed to a more frequent regimen. If a flare occurs less than 6 weeks after the last treatment, 25 percent of the maximum exposure received during the clearing phase, may be used and then proceed with the clearing schedule previously followed for this patient. (At 95 percent clearing follow regular maintenance until the optimum maintenance schedule is determined for the patient.) If more than 6 weeks have elapsed since the last treatment was given, treat patients as if they were beginning therapy insofar as exposure dosages are concerned, since their threshold for erythema may have decreased.
| Grade | Erythema Level |
|---|---|
|
0 |
No erythema |
|
1 |
Minimally perceptible erythema – faint pink |
|
2 |
Marked erythema but with no edema |
|
3 |
Fiery erythema with edema |
|
4 |
Fiery erythema with edema and blistering |
| Grade | Criteria | Percent Improvement (compared to original extent of disease) |
|---|---|---|
|
-1 |
Psoriasis worse |
0 |
|
0 |
No change |
0 |
|
1 |
Minimal improvement – slightly less scale and/or erythema |
5-20 |
|
2 |
Definite improvement – partial flattening of all plaques – less scaling and less erythema |
20-50 |
|
3 |
Considerable improvement – nearly complete flattening of all plaques but borders of plaques still palpable |
50-95 |
|
4 |
Clearing; complete flattening of plaques including borders; plaques may be outlined by pigmentation |
95 |
How is 8-MOP supplied
8-MOP Capsules, each containing 10 mg of methoxsalen (8-methoxypsoralen) are available in pink colored hard gelatin capsules of 50 (NDC 0187-0651-42), with VRX imprinted on the cap of the capsule and 600 imprinted on the body of the capsule.
References
- 1.
- Pathak, M.A., Kramer, D.M., Fitzpatrick, T.B.: Photobiology and Photochemistry of Furocoumarins (Psoralens), SUNLIGHT AND MAN: Normal and Abnormal Photobiologic Responses. Edited by M.A. Pathak, L.C. Harbor, M. Seiji et al. University of Tokyo Press. 1974, pp. 335-368.
- 2.
- Artuc, M., Stuettgen, G., Schalla, W., Schaefer, H., and Gazith, J.: Reversible binding of 5- and 8- methoxypsoralen to human serum proteins (albumin) and to epidermis in vitro: Brit. J. Dermat. 101, pp. 669-677 (1979).
- 3.
- Mandula, B.B., Pathak, M.A., Nakayama, Y., and Davidson, S.J.: Induction of mixed-function oxidases in mouse liver by psoralens., Ibid, 99, pp. 687-692 (1978).
- 4.
- Pathak, M.A., Fitzpatrick, T.B., Parrish, J.A.: PSORIASIS, Proceedings of the Second International Symposium. Edited by E.M. Farber, A.J. Cox, Yorke Medical Books, pp. 262-265 (1977).
- 5.
- Dall' Acqua, F., Marciani, S., Ciavatta, L. Rodighiero, G.: Formation of interstrand cross-linkings in the photoreactions between furocoumarins and DNA; Z Naturforsch (B), 26, pp. 561-569 (1971).
- 6.
- Cole, R.S.: Light-induced cross-linkings of DNA in the presence of a furocoumarin (psoralen), Biochem. Biophys. Acta, 217, pp. 30-39 (1970).
- 7.
- Musajo, L., Rodighiero, G., Caporale, G., Dall' Acqua, F., Marciani, S., Bordin, F., Baccichetti, F., Bevilacqua, R.: Photoreactions between Skin-Photosensitizing Furocoumarins and Nucleic Acids, SUNLIGHT AND MAN; Normal and Abnormal Photobiologic Responses. Edited by M.A. Pathak, L.C. Harber, M. Seiji et al. University of Tokyo Press, pp. 369-387 (1974).
- 8.
- Dall' Acqua, F., Vedaldi, D., Bordin, F., and Rodighiero, G.: New studies in the interaction between 8-methoxypsoralen and DNA in vitro; J. Investigative Dermat., 73, pp. 191-197 (1979).
- 9.
- Yoshikawa, K., Mori, N., Sakakibara, S., Mizuno, N., Song, P.: Photo-Conjugation of 8-methoxypsoralen with Proteins; Photochem. & Photobiol. 29, pp. 1127-1133 (1979).
- 10.
- Ortonne, J. P., MacDonald, D.M., Micoud, A., Thivolet, J.: PUVA-induced repigmentation of vitiligo: a histochemical (split-DOPA) and ultra-structural study: Brit. J. of Dermat., 101, pp. 1-12 (1979).
- 11.
- Hakim, R.E., Griffin, A.C., Knox, J.M.: Erythema and tumor formation in methoxsalen treated mice exposed to fluorescent light; Arch. Dermatol. 82, pp. 572-577 (1960).
- 12.
- Pathak, M.A., Daniels, F., Hopkins, C.E., Fitzpatrick, T.B.: Ultraviolet carcinogenesis in albino and pigmented mice receiving furocoumarins: psoralens and 8-methoxypsoralen, Nature 183, pp. 728-730 (1959).
- 13.
- Stern, R.S., Thibodeau, L.A., Kleinerman, R.A., Parrish, J.A., Fitzpatrick, T.B., and 22 Participating Investigators: Risk of Cutaneous Carcinoma in Patients Treated with Oral Methoxsalen Photochemotherapy for Psoriasis: NEJM, 300. No. 15, pp. 809-813 (1979).
- 14.
- Stern, R.S., Parrish, J.A., Zierler, S.: Skin Carcinoma in Patients with Psoriasis Treated with Topical Tar and Artificial Ultraviolet Radiation. Lancet, 1, pp. 732-735 (1980).
- 15.
- Roenigk, Jr., H.H., and 12 Cooperating Investigators: Skin Cancer in the PUVA-48 Cooperative Study of Psoriasis. Program for Forty-First Annual Meeting for The Society of Investigative Dermatology, Inc., Sheraton Washington Hotel, Washington, D.C., May 12, 13, and 14, 1980. Abstracts JID, 74, No. 4, p. 250 (April, 1980).
- 16.
- Stern et al., Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet A radiation (PUVA). The PUVA Follow-up Study. New England Journal of Medicine, 336:1041-1045, (April 10, 1997).
- 17.
- Mosher, D.B., Pathak, M.A., Harris, T.J., Fitzpatrick, T.B.: Development of Cutaneous Lesions in Vitiligo During Long-Term PUVA Therapy. Program for Forty-First Annual Meeting for The Society for Investigative Dermatology, Inc., Sheraton Washington Hotel, Washington, D.C., May 12, 13, and 14, 1980. Abstracts JID, 74, No. 4, p. 259 (April, 1980).
- 18.
- Cloud, T.M., Hakim, R., Griffin, A.C.: Photosensitization of the eye with methoxsalen. I. Acute effects; Arch. Ophthalmol. 64, pp. 346-352 (1960).
- 19.
- Cloud, T.M., Hakim, R., Griffin, A.C.: Photosensitization of the eye with methoxsalen. II. Chronic effects, Ibid, 66, pp. 689-694 (1961).
- 20.
- Freeman, R.G., Troll, D.: Photosensitization of the eye by 8-methoxypsoralen, JID, 53, pp. 449-453 (1969).
- 21.
- Lerman, S., Megaw, J., Willis, I.: Potential ocular complications from PUVA therapy and their prevention; J. Invest. Dermtat., 74, pp. 197-199 (1980).
Valeant
Pharmaceuticals North America LLC
Manufactured in Canada for:
Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807 USA
1-800-321-4576
LB0095-00
Rev. 09/13
PATIENT INFORMATION8-MOP® (ait-mahp) CAPSULES (Methoxsalen Capsules, USP, 10 mg)
IMPORTANT INFORMATION: This brochure is intended to provide you with information about the treatment of vitiligo and psoriasis. The entire brochure should be read so that you are aware of the requirements on your part to ensure the effectiveness and safety of the therapy. Any additional questions that you may have can be answered by your doctor or pharmacist. In addition, the pharmacist will have a copy of a very technical brochure entitled the "Physician's Package Insert" that you may wish to read.
- 1.
-
What Is 8-MOP® (Methoxsalen)?
8-MOP® (methoxsalen) is a drug which has been shown to be effective in the treatment of certain skin diseases when combined with exposure to a very specific kind of light. The skin diseases are vitiligo and psoriasis. In either skin disease, the use of the drug must be combined with exposure to the special light to produce effective therapy.
- 2.
-
What Is The Special Light?
Light is classified into many different parts. One part is known as ultraviolet light, which is a normal component of sunlight. Artificial or man-made light sources are now available that produce the special part of light (ultraviolet "A") necessary for the most effective therapy.
- 3.
-
What Is "PUVA"?
"PUVA" is the name of the treatment for psoriasis and stands for the use of Psoralen drug (8-MOP®) in combination with UltraViolet A light.
- 4.
-
What Is Vitiligo?
Skin color is determined by the amount of a pigment called melanin in the skin. This pigment is formed by a normal chemical reaction in the skin which is promoted by ultraviolet light (for example, tanning). In vitiligo, some areas of the skin lose their ability to produce this pigment and patches appear that have less color than your normal skin. The combination of 8-MOP® and ultraviolet light helps restore the color to these areas.
- 5.
-
Whats Is Psoriasis?
Psoriasis is a skin condition with red and scaly patches. The cause of psoriasis is not known. PUVA (8-MOP® with ultraviolet A light) is used for the treatment of severe psoriasis that has not been helped by other methods of therapy.
- 6.
-
What Should The Patient Do Before PUVA Therapy?
Certain other medicines can make you more sensitive to the combination drug and light treatment. In addition, certain other medical conditions can be aggravated by this treatment. Before starting treatment, be sure to tell your doctor if you have experienced any of the folllowing:
-
- 1.
- had a severe reaction to 8-MOP® in the past.
- 2.
- had a recent x-ray treatment or are planning any.
- 3.
- have or ever have had skin cancer.
- 4.
- have or ever have had any eye problems such as cataracts or loss of the lens of the eyes.
- 5.
- have or ever have had liver problems.
- 6.
- have or ever have had heart or blood pressure problems.
- 7.
- have any medical condition that requires you to stay out of the sun such as lupus erythematosus.
- 8.
- are taking any drugs (either prescription or nonprescription). Some drugs can increase your sensitivity to ultraviolet light either from the sun or man-made sources. Examples of such drugs include major tranquilizers, sulfa drugs for the treatment of infection or diabetes, tetracycline antibiotics, griseofulvin products, thiazide-containing diuretics (blood pressure or water elimination drugs), and certain antibacterial or deodorant soaps.
- 7.
-
How Should The Patient Take 8-MOP®?
- 9.
- The number of capsules recommended by your doctor should be taken with some food or milk according to the following schedule:
- 1.
- For vitiligo - two to four hours before ultraviolet light treatment.
- 2.
- For psoriasis - two hours before ultraviolet light treatment.
- 10.
- 8-MOP® is a potent drug. Never take more than is prescribed for you since it may result in burning and/or blistering of your skin after exposure to ultraviolet light.
- 8.
-
What Precautions Should Be Taken During And After PUVA Therapy?
- 11.
- Eye Protection - Make sure that you wear special wrap-around sunglasses that totally block or absorb ultraviolet light. Put them on immediately after taking 8-MOP® and continue wearing them for 24 hours if any light is present (even if indirect such as reflection or through window glass). Ordinary sunglasses are not adequate.
- 12.
- Skin & Lip Protection - Do not allow exposure of your skin and lips to sunlight for 8 hours after treatment. In addition, do not expose your skin to either sunlight or sun lamps (regardless of safety claims) within 24 hours of a scheduled treatment. It is advisable to wear protective clothing (hat, gloves) to cover as much of your body as possible after treatment as well as using a sunscreen product having a protection factor of at least 15 (only use after treatment).
- 9.
-
How Long Will The Treatments Last?
- 13.
- Vitiligo - May take from several months to several years to complete treatment.
- 14.
- Psoriasis - May take from six to eight weeks before lesions disappear. Maintenance treatments are usually needed to keep the disease under control.
- 10.
-
What Are The Problems Associated With Pregnancy Or Breast-Feeding?
- 15.
- Birth control methods should be employed since the effects of PUVA therapy on the unborn child are not known. If you become pregnant, inform your doctor so that he can determine wheter it is necessary for you to temporarily stop therapy.
- 16.
- Since it is not known whether 8-MOP® passes into mother's milk, it is safer not to breast feed while taking this drug.
- 11.
-
What Are The Risks Of PUVA Therapy?
- 17.
- Premature skin aging may result from prolonged PUVA therapy, especially with those individuals who tan poorly. This problem is similar to excessive exposure to sunlight.
- 18.
- There is an increased risk of developing both melanoma and non-melanoma skin cancer. This risk is greater for individuals who fall into the following categories:
- 3.
- fair skin that burns rather than tans.
- 4.
- have had prior treatment with x-rays, grenz rays, or arsenic.
- 5.
- have had coal tar and UltraViolet B (UVB) treatment.
Even though your doctor will be examining you, you should routinely and completely examine yourself for small growths on your skin or skin sores that will not heal. Immediately report such observations to your doctor.
-
- 19.
- Since studies have shown that animals with unprotected eyes have developed cataracts after PUVA therapy, you should have your eyes examined by an opthalmologist before starting PUVA therapy, after the first year of therapy, and every two years thereafter.
- 12.
-
What Are The Possible Side Effects?
- 20.
- The most common side effects of PUVA therapy are nausea, itching, and redness of the skin. The use of milk or food when ingesting the drug may prevent the nausea.
- 21.
- Tenderness or blistering of the skin may occur, but these symptoms can be helped by the use of skin products recommended by your doctor or pharmacist.
- 22.
- Less frequent side effects include depression, dizziness, headache, swelling, rash, or leg cramps.
Important: Contact your doctor if any side effect continues to bother you after 24-48 hours.
- 13.
-
What Else Should The Patient Know?
- 23.
- Remember to take 8-MOP® as directed by your doctor. If you forget to take the drug before your scheduled treatment, be sure to call your doctor to determine what he wishes you to do.
- 24.
- Remember that the drug has been prescribed specifically for you and your diagnosed condition. Do not use the drug for any other conditions nor give the drug to others even if they have similar symptoms.
- 25.
- If you think that you or anyone else has accidentally taken an overdose, stay out of the sunlight and immediately contact your poison control center, doctor, pharmacist, or nearest hospital emergency room.
- 26.
- ALWAYS KEEP THIS DRUG AND ALL OTHER DRUGS OUT OF THE REACH OF CHILDREN.
- 27.
- Store at 25°C (77°F); excursions permitted to 15°C-30°C (59°F-86°F).
PRINCIPAL DISPLAY PANEL - 10 mg Bottle Label
NDC 0187-0651-42
Rx Only
8-MOP®
(Methoxsalen Capsules, USP)
|
CAUTION: 8-MOP® Capsules (Methoxsalen
|
10 mg
50 Capsules
VALEANT
Pharmaceuticals North America LLC
| 8-MOP
methoxsalen capsule, gelatin coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Valeant Pharmaceuticals North America LLC (042230623) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Valeant Pharmaceuticals International, Inc. | 253292734 | MANUFACTURE(0187-0651) | |
More about 8-MOP (methoxsalen)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: psoralens
- Breastfeeding