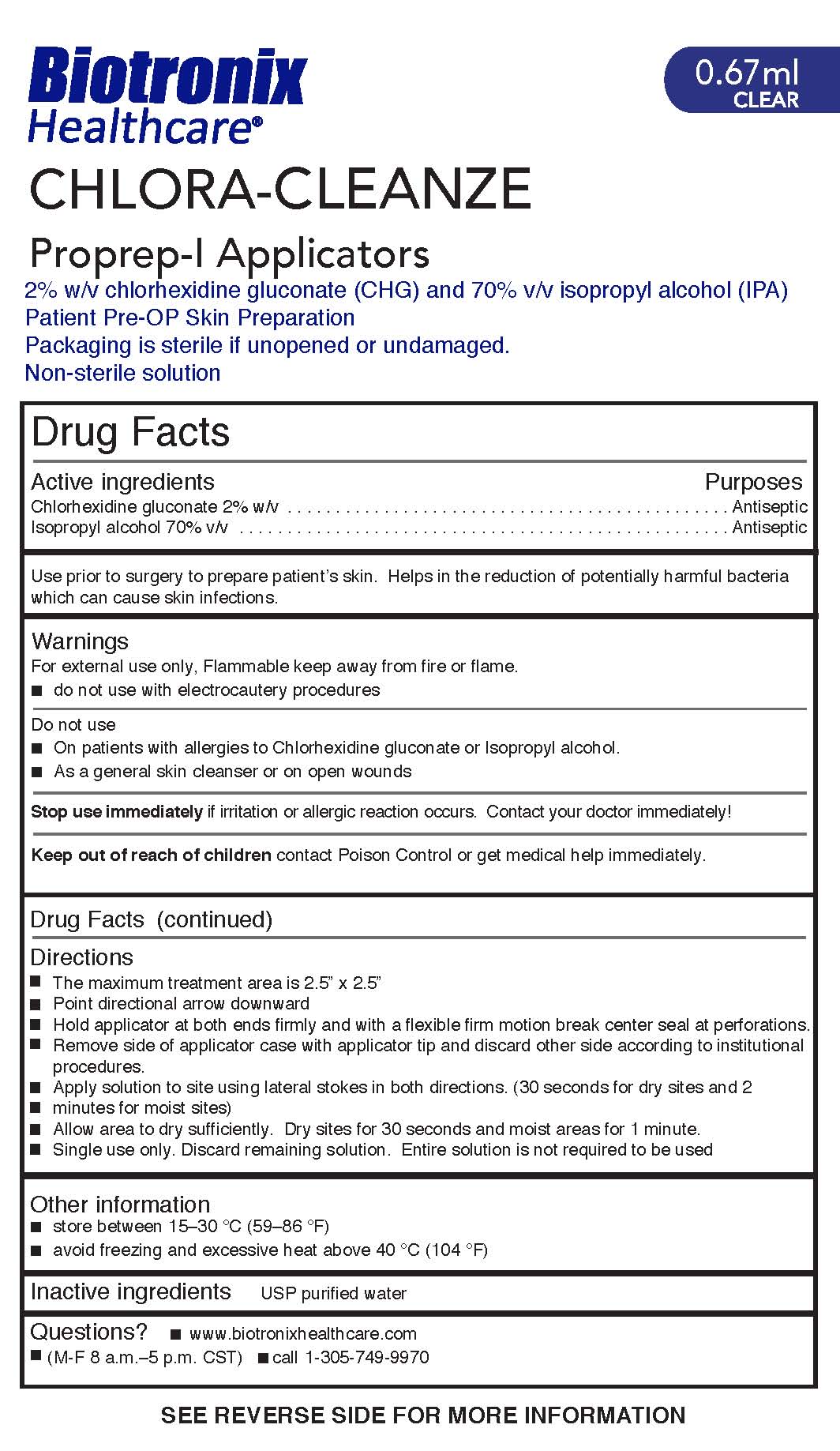
CHLORA-CLEANZE
Dosage form: swab
Ingredients: ISOPROPYL ALCOHOL 0.7mL in 1mL, CHLORHEXIDINE GLUCONATE 20mg in 1mL
Labeler: Biotronix Healthcare Industries, Inc.
NDC code: 71389-001
Medically reviewed by Drugs.com. Last updated on Jun 25, 2025.
Active ingredients
Chlorhexidine gluconate 2% w/v
Isopropyl alcohol 70% v/v
Purposes
Antiseptic
Warnings
For external use only. Flammable keep away from fire or flame.
- do not use with electrocautery procedures
Do not use
- On patients with allergies to Chlorhexidine gluconate or Isopropyl alcohol
- As a general skin cleanser or on open wounds
Stop use immediately if irritation or allergic reaction occurs. Contact your doctor immediately!
Directions
- The maximum treatment area is 2.5” x 2.5”
- Point directional arrow downward
- Hold applicator at both ends firmly and with a flexible firm motion break center seal at perforations.
- Remove side of applicator case with applicator tip and discard other side according to institutional
procedures.
- Apply solution to site using lateral stokes in both directions. (30 seconds for dry sites and 2
minutes for moist sites)
- Allow area to dry sufficiently. Dry sites for 30 seconds and moist areas for 1 minute.
- Single use only. Discard remaining solution. Entire solution is not required to be used
Inactive ingredients USP purified water
Use prior to surgery to prepare patient’s skin. Helps in the reduction of potentially harmful bacteria which can cause skin infections.
Other information
■ store between 15–30 °C (59–86 °F)
■ avoid freezing and excessive heat above 40 °C (104 °F)
Keep out of reach of children contact Poison Control or get medical help immediately.

| CHLORA-CLEANZE
chlorhexidine gluconate and isopropyl alcohol swab |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - Biotronix Healthcare Industries, Inc. (065762392) |
| Registrant - US MED PHARM SUPPLIES, INC. (831533109) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Longood Medicine (Beijing) Co., Ltd. | 529553064 | manufacture(71389-001) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
