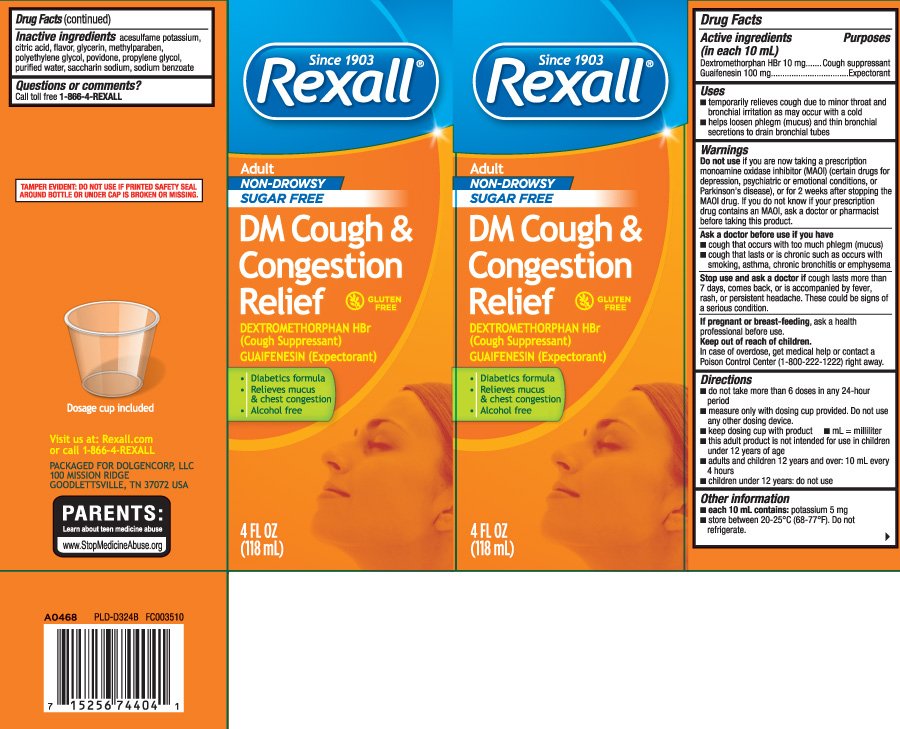
DM Cough and Congestion Relief Adult non-Drowsy Sugar Free
Dosage form: liquid
Ingredients: DEXTROMETHORPHAN HYDROBROMIDE 10mg in 10mL, GUAIFENESIN 100mg in 10mL
Labeler: Dolgencorp, Inc. (DOLLAR GENERAL & REXALL)
NDC code: 55910-584
Medically reviewed by Drugs.com. Last updated on Dec 2, 2024.
Dextromethorphan HBr 10 mg
Guaifenesin 100 mg
Cough suppressant
Expectorant
• temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
• helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask
a doctor or pharmacist before taking this product.
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask
a doctor or pharmacist before taking this product.
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
- do not takemore than 6 doses in any 24-hour period
- measure only with dosing cup provided. Do not use any other dosing device.
- keep dosing cup with product
- mL = milliliter
- this adult product is not intended for use in children under 12 years of age
- children under 12 years: do not use
- each 10 mL contains: potassium 5 mg
- store between 20-25ºC (68-77ºF). Do not refrigerate.
acesulfame potassium, citric acid, flavors, glycerin, methylparaben, polyethylene glycol, povidone, propylene glycol, purified water, saccharin sodium, sodium benzoate
Call toll free 1-866-4-REXALL
Adult
NON-DROWSY SUGAR FREE
DM Cough & Congestion Relief
DEXTROMETHORPHAN HBr (Cough Suppressant)
GUAIFENESIN (Expectorant)
GLUTEN FREE
- Diabetics formula
- Relieves mucus & chest congestion
- Alcohol free
FL OZ (mL)
Dosage cup included
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL AROUND BOTTLE OR UNDER CAP IS BROKEN OR MISSING.
Visit us at: Rexall.com
PACKAGED FOR DOLGENCORP, LLC
100 MISSION RIDGE
GOODLETTSVILLE, TN 37072 USA
Rexall DM Cough & Congestion Relief
| DM COUGH AND CONGESTION RELIEF
ADULT NON-DROWSY SUGAR FREE
dextromethorphan hydrobromide, guaifenesin liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Dolgencorp, Inc. (DOLLAR GENERAL & REXALL) (068331990) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
