Calcemin Advance
Dosage form: tablet, film coated
Ingredients: CALCIUM CARBONATE 500mg, PREVITAMIN D3 3mg, MAGNESIUM OXIDE 40mg, ZINC OXIDE 7.5mg, SODIUM BORATE 2.45mg
Labeler: Bayer HealthCare LLC.
NDC code: 0280-0272
Medically reviewed by Drugs.com. Last updated on May 19, 2025.
Drug Facts
Drug Facts
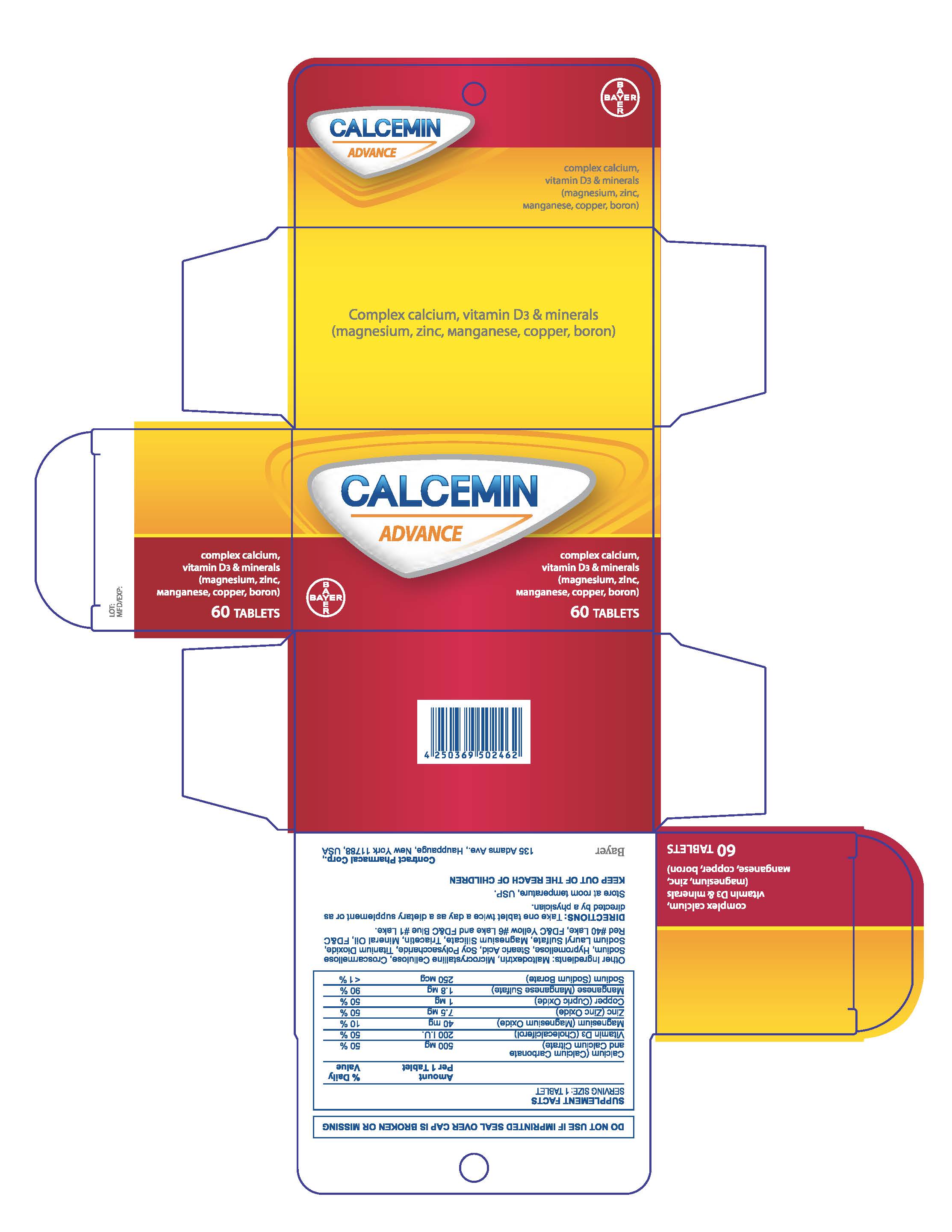
| CALCEMIN ADVANCE
calcium citrate tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |
Document Id: a6baf3a0-dd16-5a99-e053-2a95a90a595a
Set id: 3161469e-6402-607a-e054-00144ff88e88
Version: 3
Bayer HealthCare LLC.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
