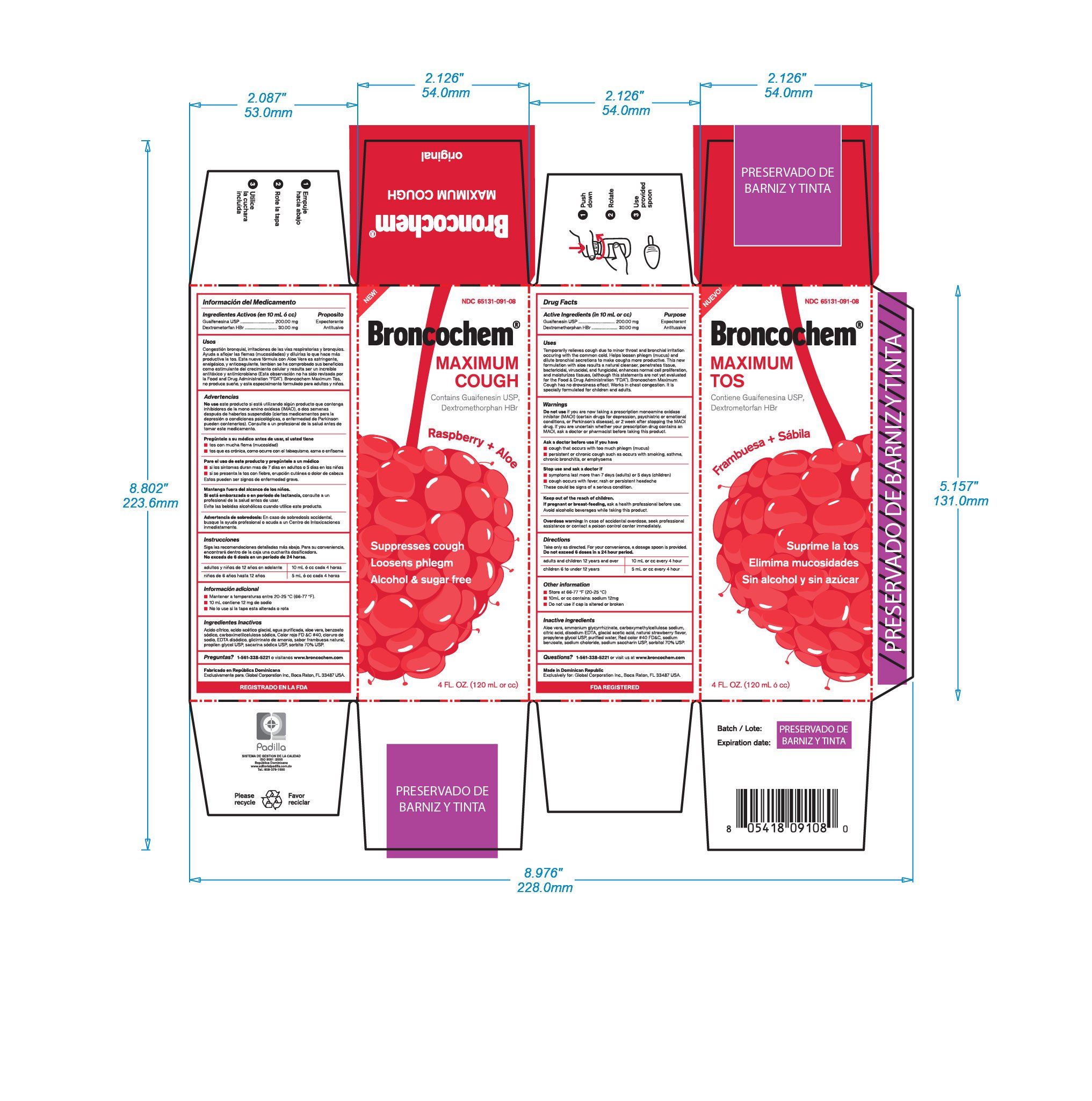
BRONCOCHEM MAXIMUM COUGH
Dosage form: syrup
Ingredients: DEXTROMETHORPHAN HYDROBROMIDE 30mg in 10mL, GUAIFENESIN 200mg in 10mL
Labeler: LABORATORIO MAGNACHEM INTERNATIONAL SRL
NDC code: 65131-091
Medically reviewed by Drugs.com. Last updated on Dec 9, 2024.
Do not take this product for persitent or chronic cough such as occurs with smoking, asthma, or emphysema or if cough is accompainied with excessive phlegm (mucus) unless directed by a doctor. Persistent cough may be the sign of a serious condition. Stop use and dask a doctor if symptoms persist or last more than 5 days (children) or 7 days (adults), tends to recur, is accompained or followed by fever, headache, rash, swelling, nausea or vomiting, consult a doctor. Do not take this product if you are hypersensitive to any of the ingredients. As with any drug, if you are pregnant or nursing a baby, seek the advice of a health professional before using this product. Avoid alcoholic beverages while taking this product.
Guaifenesin
Dextromethorphan HBr
Expectorant
Antitussive
In case of accidental overdose, seek professional assistance or contact a poison control center immediately
Temporarily relieves cough due to minor throat and bronchial irritation occuring with common cold.
Helps loosen phlegm (mucus) and dilute bronchial secretions to make coughs more productive.
This new formulation with aloe results a natural cleanser, penetrates tissue, bactericidal, viruscidal, and fungicidal, enhances normal cell proliferation and moisturizes tissues (this statements are not yet evaluated by the Food and Drugs Administration "FDA")
Broncochem Maximum Cough has not drowsiness effect.
Work in chest congestion, its specially formulated for children and adults.
Do not exceed 6 doses in a 24 hour period
Adults and children 12 years and over: (10mL or cc) every 4 hours
Children 6 years up to 12 years: (5mL or cc) every 4 hours
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI), (certain drugs for depression, psychiatric or emotional conditions or Parkinsons disease), or 2 weeks after stopping the MAOI drug. Id you are uncertain ehether your prescription drug contains an MAOI, consult a health professional before taking this product
Aloe Vera, Ammonium Glycyrrhizinate, Citric Acid, Disodium HEDTA, Glacial Acetic Acid, Strawberry flavor, Propylene Glycol, Purified Water, FD&C Red 40, Sodium Benzoate, Sodium Chloride, Sodium Saccharin, Sorbitol, Carboxymethylcellulose sodium, Total Sodium content: 12mg (in 10mL or cc)
| BRONCOCHEM
MAXIMUM COUGH
dextromethorphan hbr-gyaifenesin syrup |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - LABORATORIO MAGNACHEM INTERNATIONAL SRL (871446100) |
| Registrant - LABORATORIO MAGNACHEM INTERNATIONAL SRL (871446100) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LABORATORIO MAGNACHEM INTERNATIONAL SRL | 871446100 | manufacture(65131-091) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
