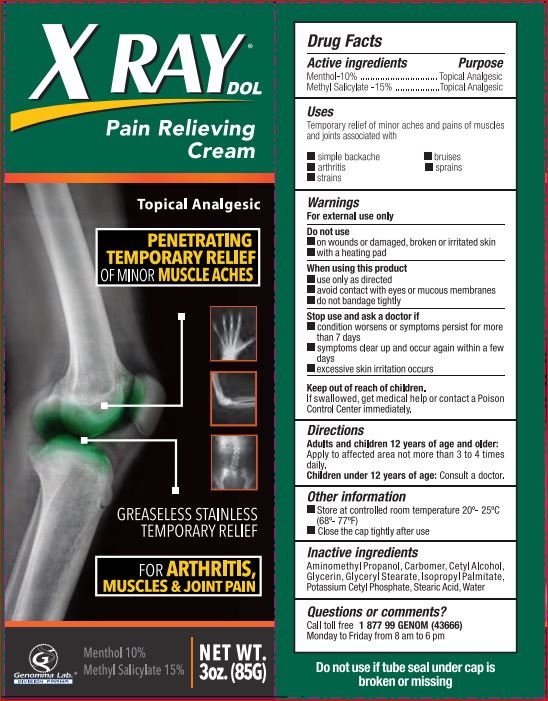
X Ray Pain Relieving
Dosage form: cream
Ingredients: Menthol 10g in 100mL, Methyl Salicylate 15g in 100mL
Labeler: Genomma Lab
NDC code: 50066-501
Medically reviewed by Drugs.com. Last updated on Mar 26, 2025.
Menthol-10% .................................... Topical Analgesic
Methyl Salicylate -15% .....................Topical Analgesic
Temporary relief of minor aches and pains of muscles
and joints associated with
- simple backache
- bruises
- arthritis
- sprains
- strains
For external use only
Do not use
- on wounds or damaged, broken or irritated skin
- with heating pad
When using this product
- use only as directed
- avoid contact with eyes or mucous membranes
- do not bandage tightly
Stop use and ask a doctor if
- condition worsens or symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- excessive skin irritation occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately
Adults and children 12 years of age and older: Apply to affected area not more than 3 to 4 times daily.
Children under 12 years of age: Consult a doctor.
Aminomethyl Propanol,Carbomer,Cetyl Alcohol,Glycerin,Glyceryl Stearate,Isopropyl Palmitate,Potassium Cetyl Phosphate,Stearic Acid,Water
| X RAY PAIN RELIEVING
menthol - 10.00% methyl salicylate - 15.00% cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Genomma Lab (832323534) |
| Registrant - Product Quest Mfg. (927768135) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Product Quest Mfg. | 927768135 | manufacture(50066-501) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
