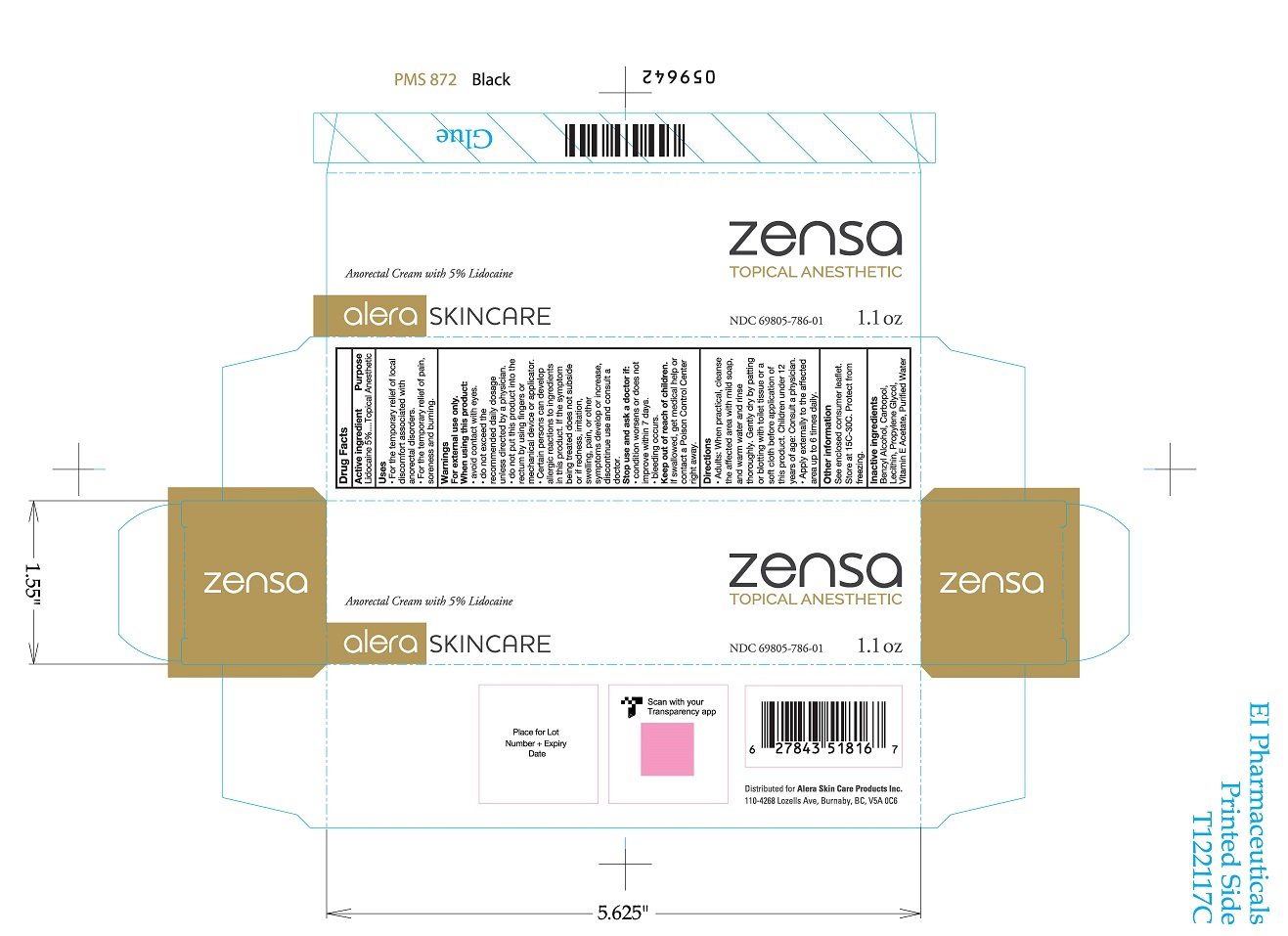
Zensa
Dosage form: cream
Ingredients: LIDOCAINE 5g in 100g
Labeler: Alera Skin Care Products Inc.
NDC code: 69805-786
Medically reviewed by Drugs.com. Last updated on Sep 2, 2024.
Active ingredient
Lidocaine 5%
Purpose
Topical Anaesthetic
Uses
- For the temporary relief of local discomfort associated with anorectal disorders
- For the temporary relief of pain, soreness, and burning
Warnings
For external use only.
When using this product
- avoid contact with eyes
- do not exceed the recommended daily dosage unless directed by a physician
- do not put this product into the rectum by using fingers or any mechanical device or applicator
- certain persons can develop allergic reactions to ingredients in this product. If the symptom being treated does not subside or if redness, irritation, swelling, pain, or other symptoms develop or increase, discontinue use and consult a doctor
Stop use and ask a doctor if
- condition worsens or does not improve within 7 days
- bleeding occurs
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
Directions
- Adults: when practical, cleanse the affected area with mild soap, and warm water and rinse thoroughly. Gently dry by patting or blottig with toilet tissue or a soft cloth before application of this product. Children under 12 years of age: consult a physician.
- Apply externally to the affected area up to 6 times daily
Other information
See enclosed consumer leaflet.
Store at 15°C to 30°C. Protect from freezing
Inactive ingredients
Benzyl Alcohol, Carbopol, Lecithin, Propylene Glycol, Vitamin E Acetate, Purified Water
NDC 69805-786-01
ZENSA
TOPICAL ANESTHETIC CREAM - ANORECTAL
WITH 5% LIDOCAINE
30 g
| ZENSA
lidocaine cream |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Alera Skin Care Products Inc. (202686093) |
| Registrant - Alera Skin Care Products Inc. (202686093) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Allure Labs, Inc | 926831603 | manufacture(69805-786) | |
Document Id: a45d81e8-b36c-47a0-a526-220ce7055338
Set id: f56146b6-3c11-4608-a6ab-6142f27e9ac3
Version: 2
Alera Skin Care Products Inc.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
