Dermal Wound Cleanser
Dosage form: spray
Ingredients: BENZETHONIUM CHLORIDE 1.3g in 981mL
Labeler: Smith & Nephew Medical Ltd.
NDC code: 69740-490
Medically reviewed by Drugs.com. Last updated on Jul 28, 2025.
benzethonium chloride 0.13%
First aid antiseptic
- first aid to help reduce the risk of infection in minor cuts, scrapes and burns
- for washing small superficial wounds
- aids in the removal of foreign materials such as dirt and debris
- For external use only
- Do not use in the eyes or apply over large areas of the body
- In case of deep or puncture wounds, animal bites, or serious burns, contact a doctor
- Stop use and contact a doctor if the condition persists or gets worse. Do not use longer than 1 week unless directed by a doctor
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately
- clean the affected area
- apply a small amount of this product on the area 1 to 3 times daily
- rinse as per normal protocol
- cover wound with sterile dressing or bandage as needed
- if applying dressing or bandage, let dry first
- contains antimicrobial ingredient
benzyl alcohol, citric acid, disodium EDTA, glycerin, polyquaternium-10, polysorbate 20, sodium citrate, water
1 800 876-1261
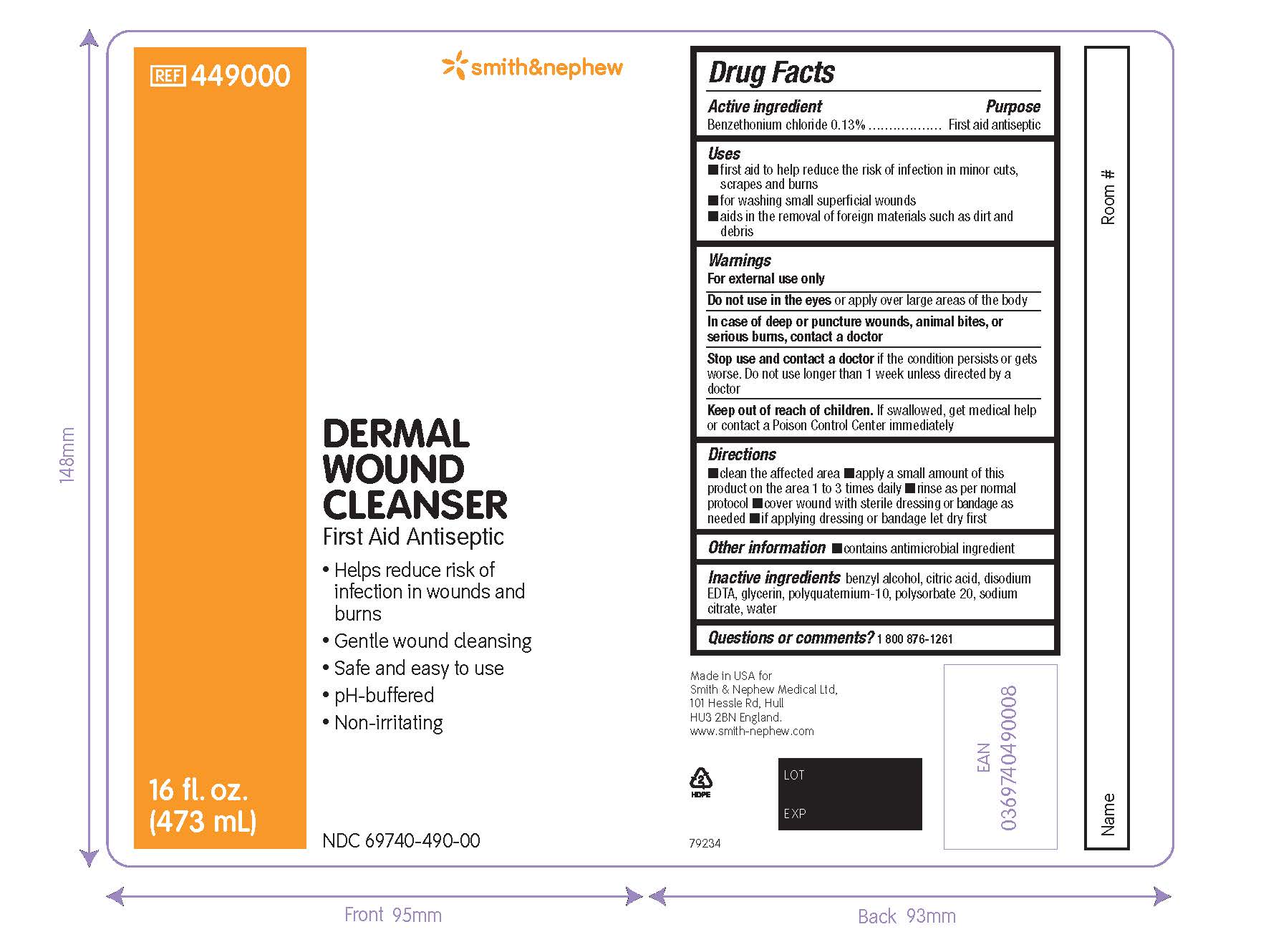
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - DERMAL WOUND CLEANSER BOTTLE, SPRAY (473mL)
Smith&Nephew
#449000
NDC 69740-490-00
Dermal
Wound
Cleanser
First Aid Antiseptic
- Helps reduce risk of infection in wounds and burns
- Gentle wound cleansing
- Safe and easy to use
- pH-buffered
- Non-irritating
16 fl. oz. (473mL)
Made in the USA for Smith & Nephew Medical Ltd
101 Hessle Road
Hull
HU3 2BN
England
www.smith-nephew.com
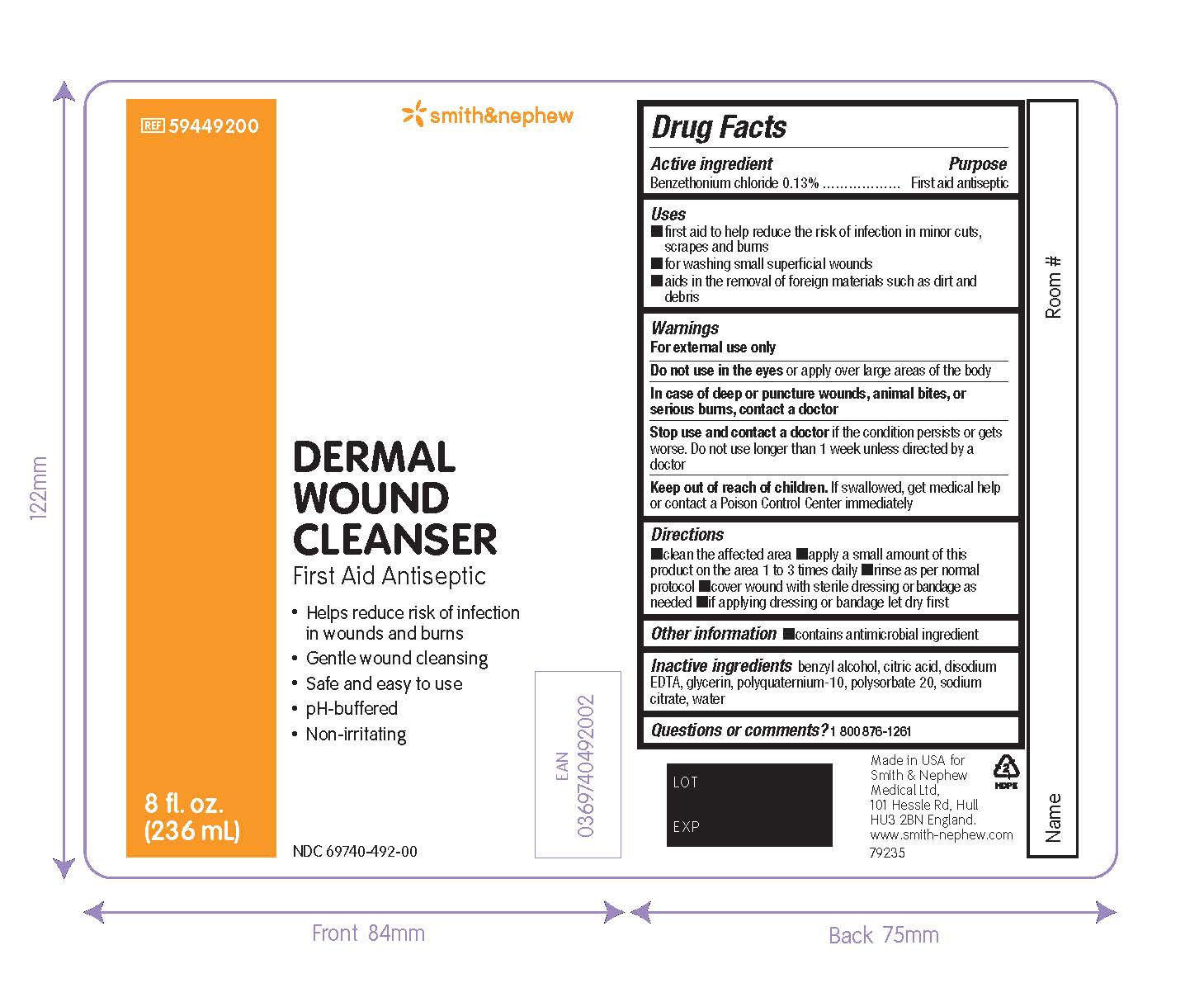
Smith&Nephew
#59449200
NDC 69740-492-00
Dermal
Wound
Cleanser
First Aid Antiseptic
- Helps reduce risk of infection in wounds and burns
- Gentle wound cleansing
- Safe and easy to use
- pH-buffered
- Non-irritating
8 fl. oz. (236mL)
Made in the USA for Smith & Nephew Medical Ltd
101 Hessle Road
Hull
HU3 2BN
England
www.smith-nephew.com
| DERMAL WOUND CLEANSER
benzethonium chloride spray |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| DERMAL WOUND CLEANSER
benzethonium chloride spray |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Smith & Nephew Medical Ltd. (216344051) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Swiss-American CDMO, LLC | 080170933 | MANUFACTURE(69740-490, 69740-492) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
