DR CLINIC 75% ALCOHOL WIPES
Dosage form: cloth
Ingredients: ALCOHOL 75mL in 100g
Labeler: MAKYAJ KOZMETIK INSAAT SANAYI VE TICARET LIMITED SIRKETI
NDC code: 76725-012
Medically reviewed by Drugs.com. Last updated on Sep 1, 2025.
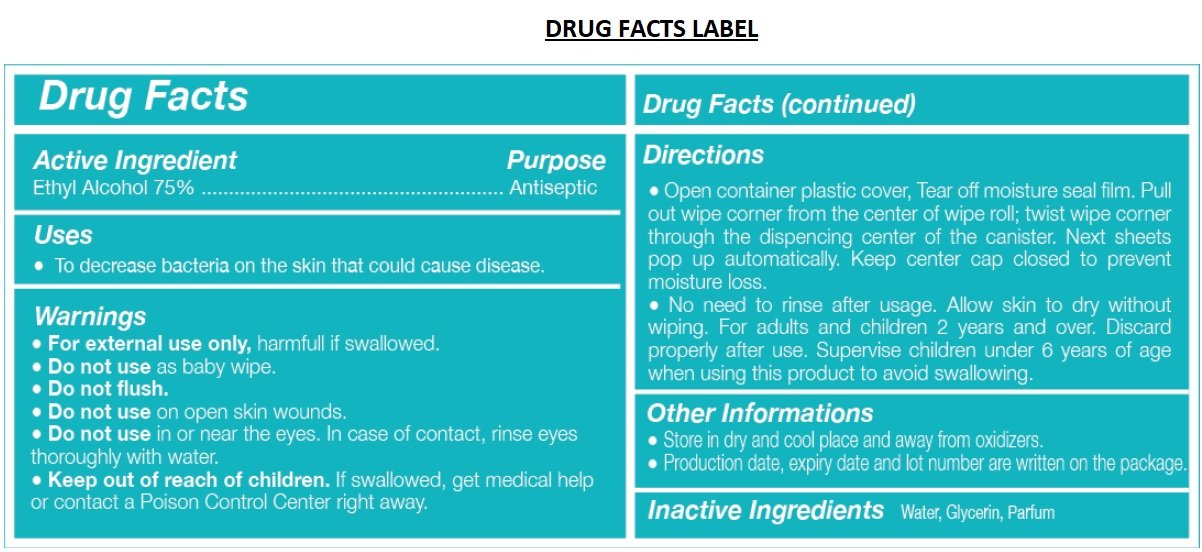
Ethyl Alcohol 75%
Antiseptic
• To decrease bacteria on the skin that could cause disease.
• For external use only, harmful if swallowed.
• Do not use as baby wipe.
• Do not flush.
• Do not use on open skin wounds.
• Do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
• Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
• Open container plastic cover, Tear off moisture seal film. Pull out wipe corner from the center of wipe roll; twist wipe corner through the dispensing center of the canister. Next sheets pop up automatically. Keep center cap closed to prevent moisture loss.
• No need to rinse after usage. Allow skin to dry without wiping. For adults and children 2 years and over. Discard properly after use. Supervise children under 6 years of age when using this product to avoid swallowing.
• Store in dry and cool place and away from oxidizers.
• Production date, expiry date and lot number are written on the package.
Inactive Ingredients Water, Glycerin, Parfum
ANTIVIRUS
ANTI-FUNGAL
Kills 99.9% Bacteria & Germs
75% Alcohol Concentration
99.9% Efficient Sterilization
Non-Woven Skin
Moisturizes and Does Not Dry
BLEACH FREE
Made in Turkey
Distributed by IVI
200 Blydenburg Rd.
Unit 3 -Islandia NY 11749
Email:info@drclinicusa.com
Phone: (800)686-5044


| DR CLINIC 75% ALCOHOL WIPES
ethyl alcohol cloth |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
| Labeler - MAKYAJ KOZMETIK INSAAT SANAYI VE TICARET LIMITED SIRKETI (504546402) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| MAKYAJ KOZMETIK INSAAT SANAYI VE TICARET LIMITED SIRKETI | 504546402 | manufacture(76725-012) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
