Hand Sanitizer Soothing Gel
Dosage form: liquid
Ingredients: ALCOHOL 70.473mL in 100mL
Labeler: OXYGEN DEVELOPMENT, LLC
NDC code: 61354-010
Medically reviewed by Drugs.com. Last updated on May 29, 2025.
Ethyl Alcohol 70% v/v. Purpose: Antimicrobial
Antimicrobial, Hand Sanitizer
Hand Sanitizer to help reduce bacteria on the skin.
Flammable. Keep away from heat or flame. For external use only
When using this product do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash appears and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- Place enough product in your palm to cover hands and rub hands together until dry.
- Children under 6 years of age should be supervised when using this product.
- Store below 110F (43C).
- May discolor certain fabric surfaces.
Water/Aqua,Glycerin, Carbomer, Aminomethyl Propanol, Aloe Barbadensis Leaf Juice.
925 mL NDC: 61354-010-01

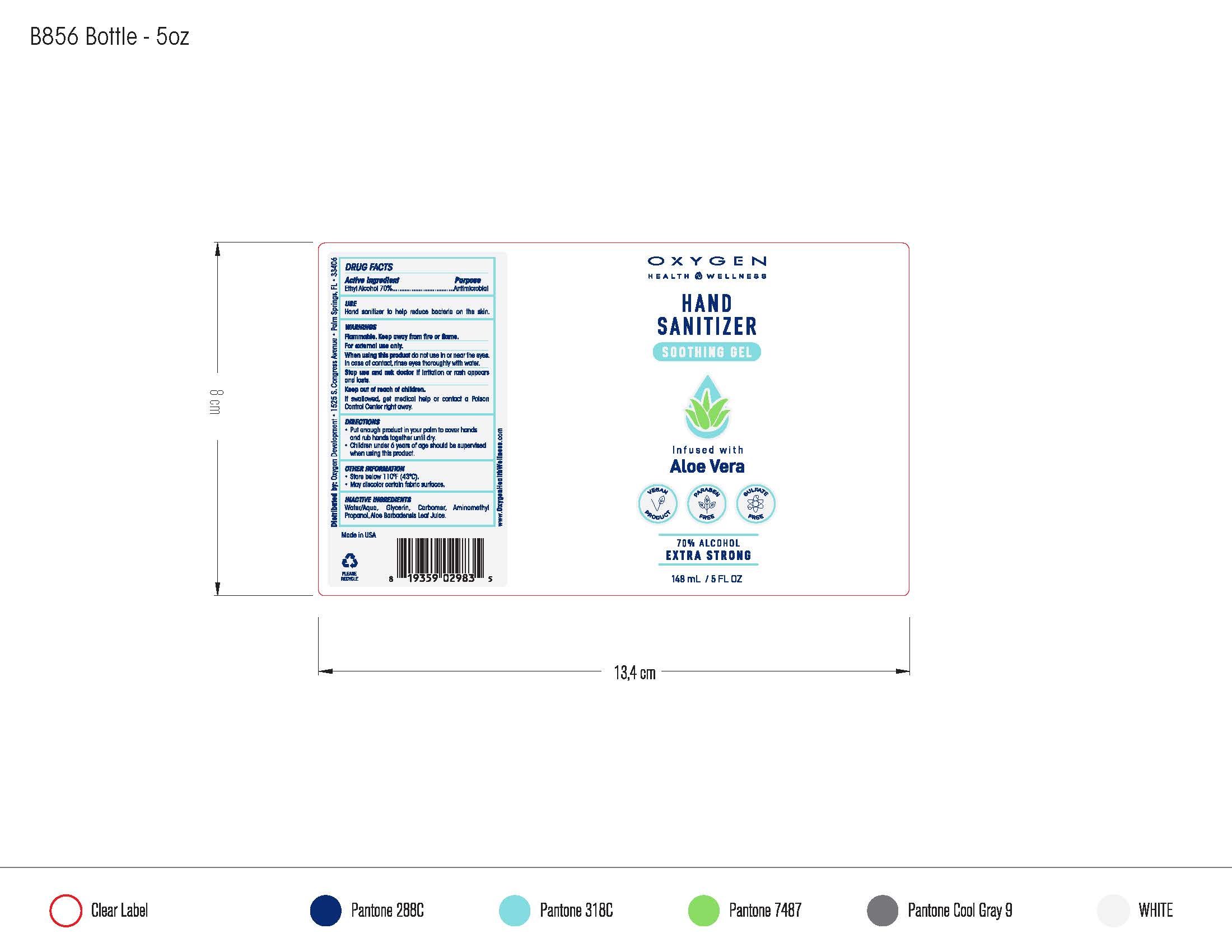 148 mL NDC:61354-010-02
148 mL NDC:61354-010-02
 55 mL NDC:61354-010-03
55 mL NDC:61354-010-03
| HAND SANITIZER SOOTHING GEL
alcohol liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - OXYGEN DEVELOPMENT, LLC (137098492) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| OXYGEN DEVELOPMENT, LLC | 137098492 | manufacture(61354-010), label(61354-010) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
