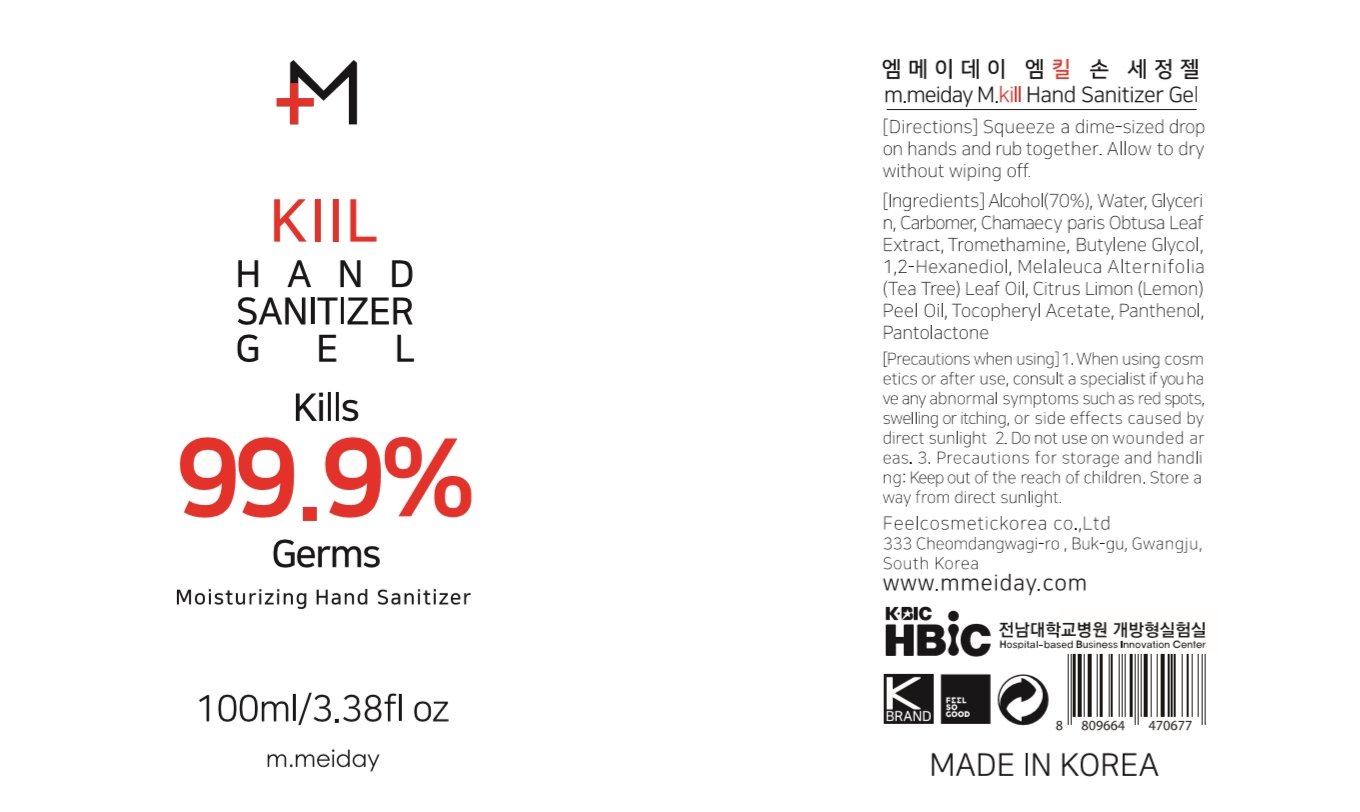
m.meiday M.kill Hand Sanitizer Gel
Dosage form: gel
Ingredients: ALCOHOL 70mL in 100mL
Labeler: FEEL COSMETIC KOREA CO., LTD
NDC code: 74454-319
Medically reviewed by Drugs.com. Last updated on Jul 28, 2025.
Alcohol 70%
Antiseptic
Water, Glycerin, Carbomer, Chamaecy paris Obtusa Leaf Extract, Tromethamine, Butylene Glycol, 1,2-Hexanediol, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Citrus Limon (Lemon) Peel Oil, Tocopheryl Acetate, Panthenol, Pantolactone
Squeeze a dime-sized drop on hands and rub together. Allow to dry without wiping off.
For external use only. Flammable. Keep away from heat or flame.
in children less than 2 months of age
on open skin wounds
keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
if irritation or rash occurs. These may be signs of a serious condition.
If swallowed, get medical help or contact a Poison Control Center right away.
Hand Sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
Store between 15-30C (59-86F)
Avoid freezing and excessive heat above 40C (104F)
| M.MEIDAY M.KILL HAND SANITIZER GEL
alcohol gel |
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - FEEL COSMETIC KOREA CO., LTD (694854046) |
| Registrant - FEEL COSMETIC KOREA CO., LTD (694854046) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| FEEL COSMETIC KOREA CO., LTD | 694854046 | manufacture(74454-319) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.