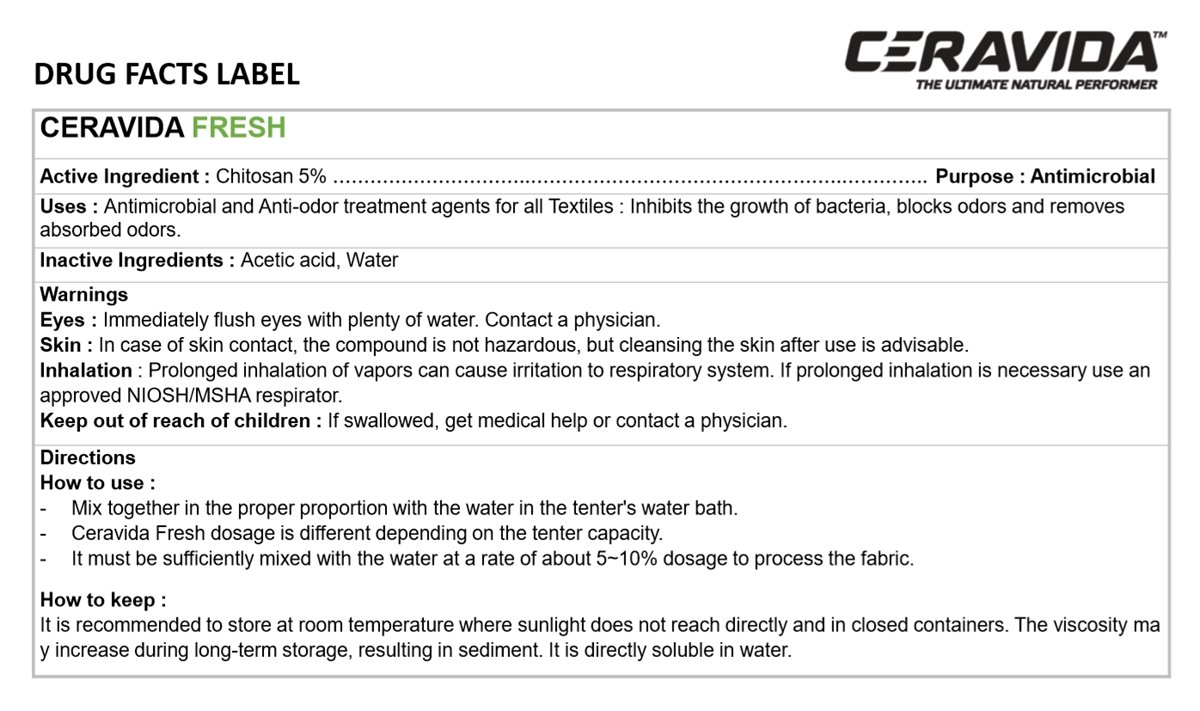
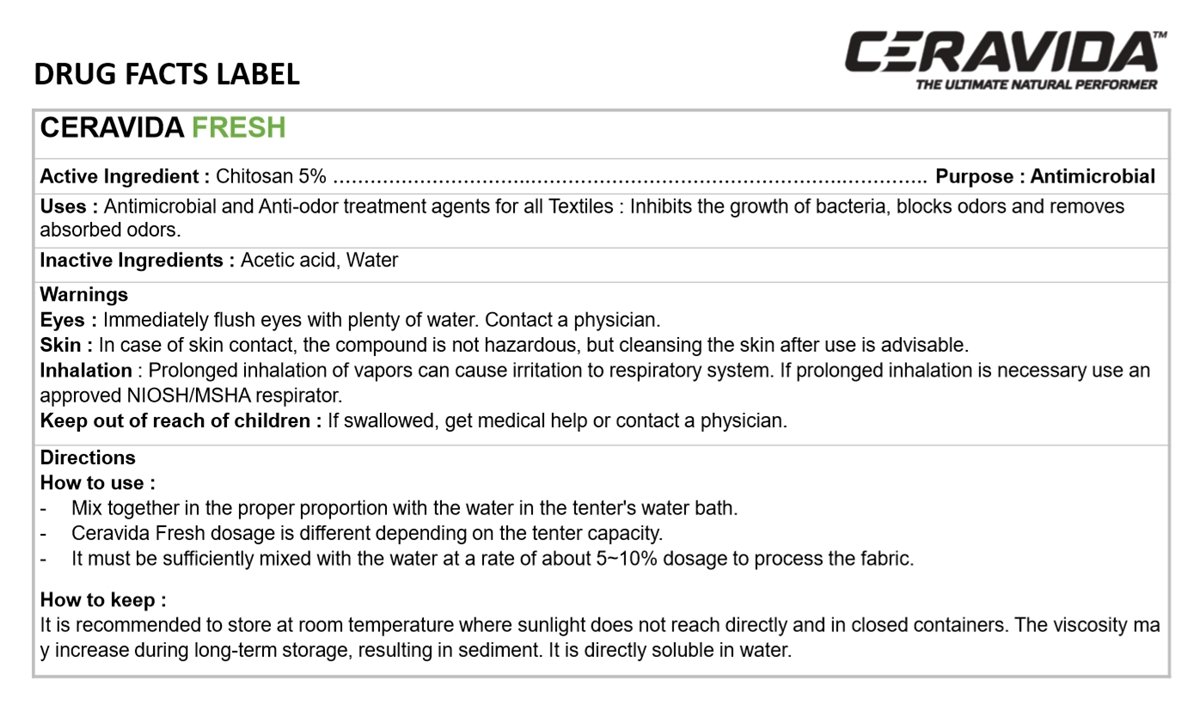
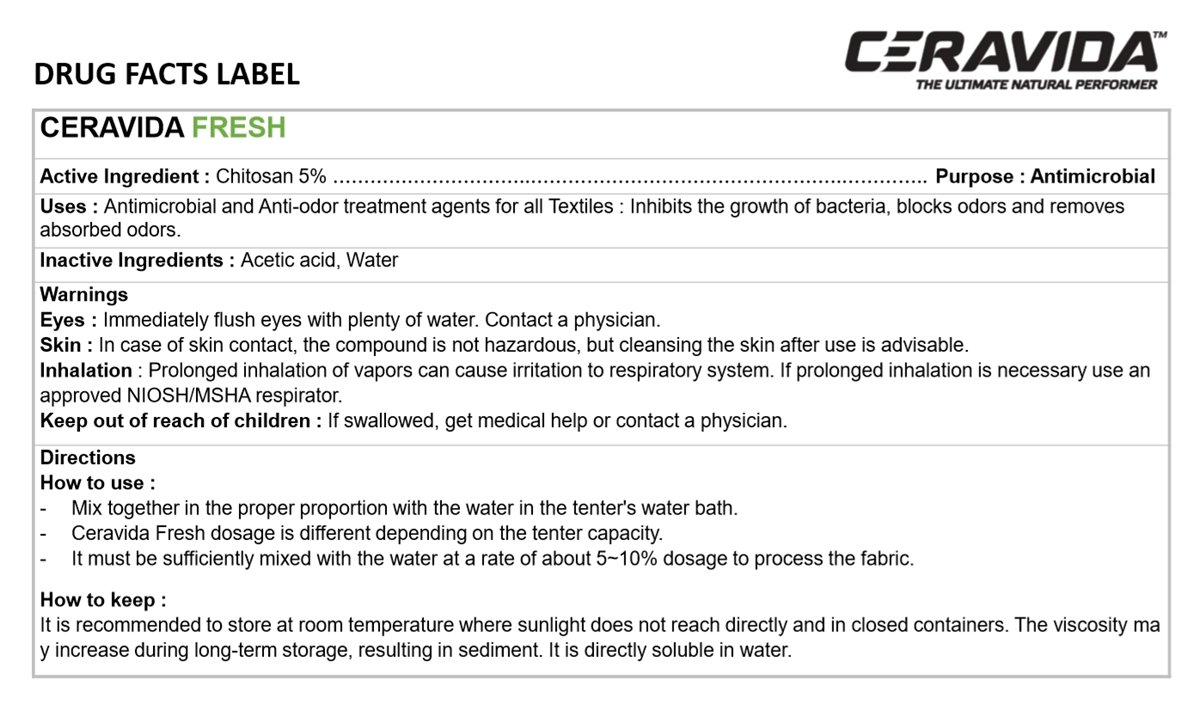
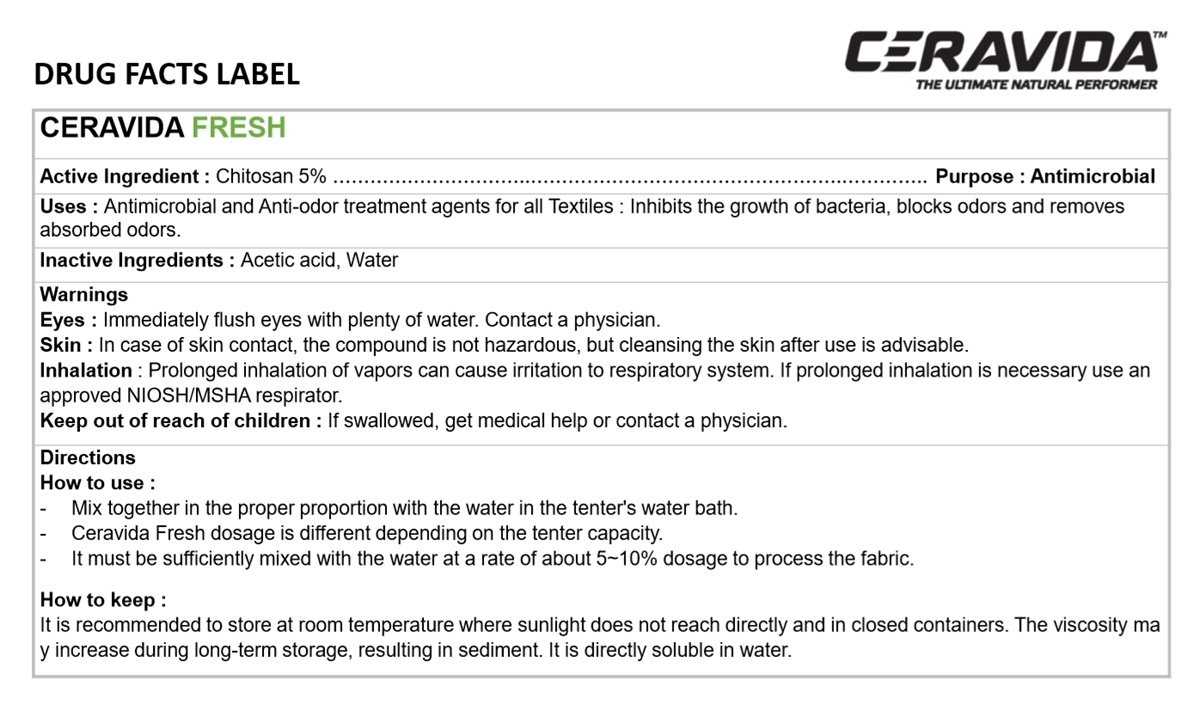
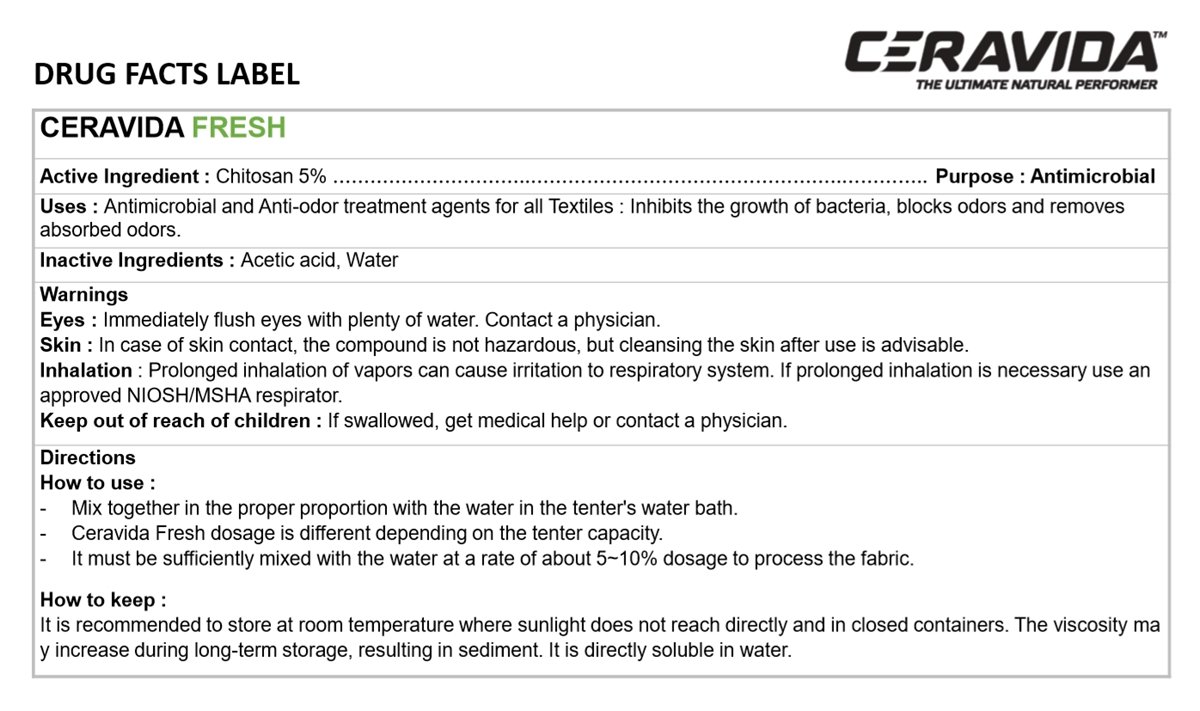
CERAVIDA FRESH
Dosage form: liquid
Ingredients: POLIGLUSAM 5kg in 100kg
Labeler: G.CLO CO., LTD
NDC code: 78543-100
Medically reviewed by Drugs.com. Last updated on May 23, 2025.
Chitosan 5%
Antiseptic
Antimicrobial and Anti-odor treatment agents for all Textiles: Inhibits the growth of bacteria, blocks odors and removes absorbed odors.
Acetic acid, Water
Immediately flush eyes with plenty of water. Contact a physician.
Inhalation
In case of skin contact, the compound is not Hazardous, but cleansing the skin after use is advisable.
Prolonged inhalation of vapors can cause irritation to respiratory system. If prolonged inhalation is necessary use an approved NIOSH/MSHA respirator.
If swallowed, get medical help or contact a physician.
How to use
- Mix together in the proper proportion with the water in the tenter's water bath.
- Ceravida Fresh dosage is different depending on the tenter capacity.
- It must be sufficiently mixed with the water at a rate of about 5-10% dosage to process the fabric.
It is recommended to store at room temperature where sunlight does not reach directly and in closed containers. The viscosity may increase during long-term storage, resulting in sediment. It is directly soluble in water.





| CERAVIDA FRESH
chitosan liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - G.CLO CO., LTD (690058546) |
| Registrant - G.CLO CO., LTD (690058546) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| G.CLO CO., LTD | 690058546 | manufacture(78543-100) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
