Puricia
Dosage form: liquid
Ingredients: ALCOHOL 70mL in 100mL
Labeler: PHARMABERG INC.
NDC code: 77302-001
Medically reviewed by Drugs.com. Last updated on May 1, 2025.
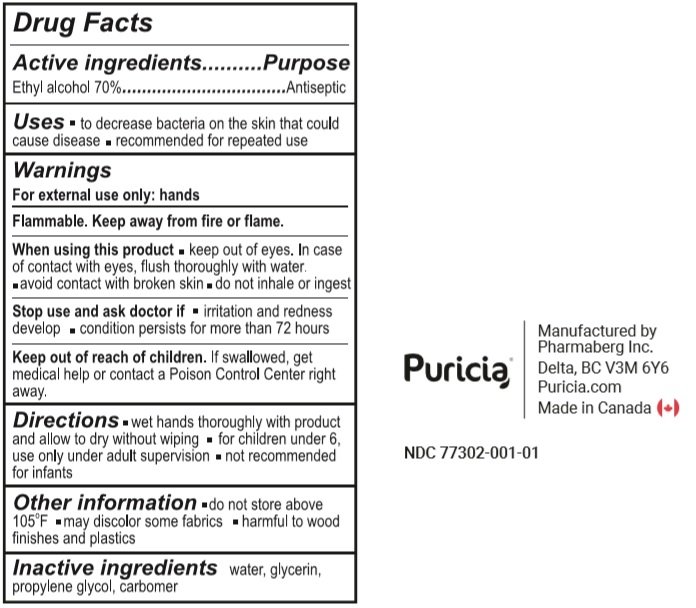
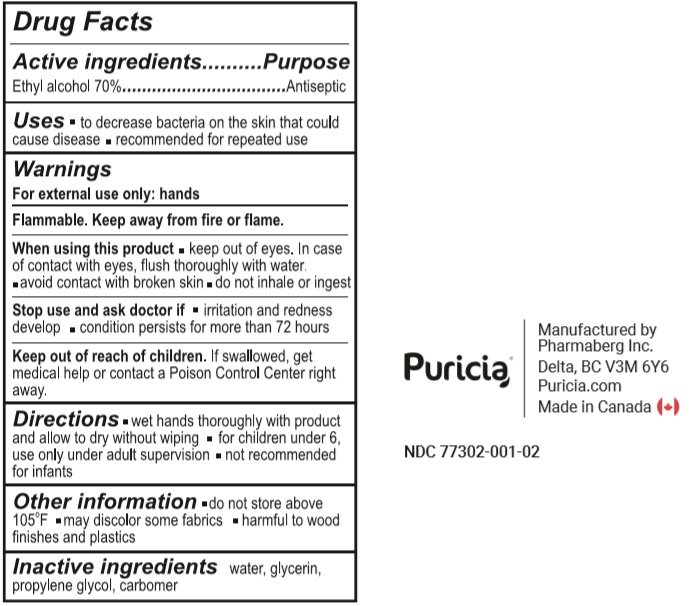
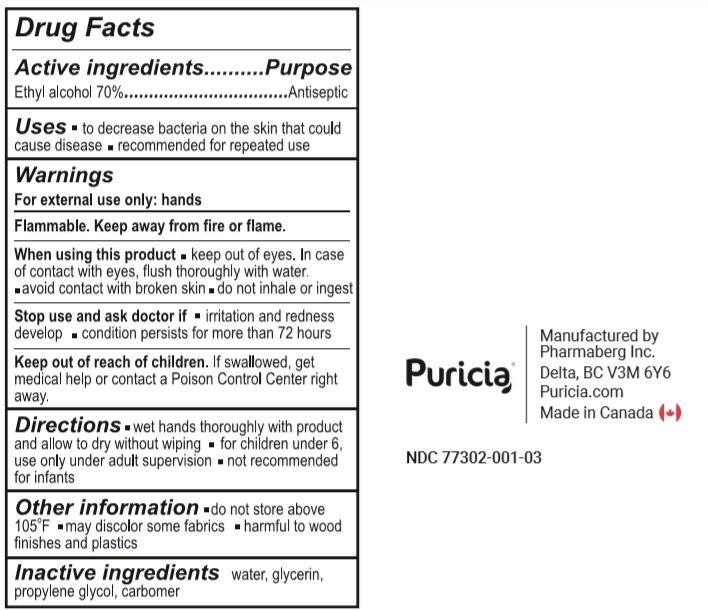
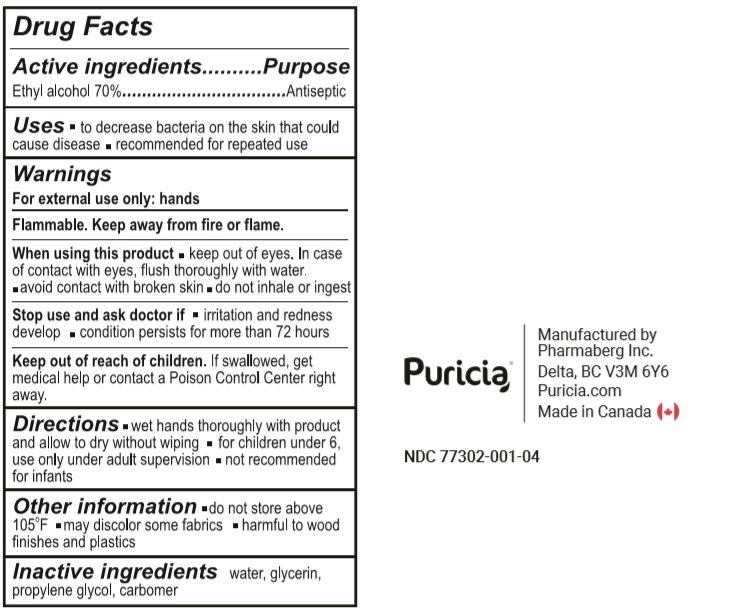
Ethyl alcohol 70%
Antiseptic
- To decrease bacteria on the skin that could cause disease.
- Recommended for repeated use.
For external use only: hands
Flammable. Keep away from fire or flame.
When using this product
- keep out of eyes. In case of contact with eyes, flush thoroughly with water.
- avoid contact with broken skin.
- do not inhale or ingest.
Stop use and ask a doctor if
- irritation or redness develop.
- condition persists for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Centre right away
- wet hands thoroughly with product and allow to dry without wiping.
- for children under 6, use only under adult supervision
- not recommended for infants.
- do not store above 105°F
- may discolor some fabrics.
- harmful to wood finishes and plastics
Water, Glycerin, Propylene Glycol, Carbomer.
60 ml NDC: 77302-001-01
 60 ml NDC: 77302-001-01
60 ml NDC: 77302-001-01
100 ml NDC: 77302-001-02
 100 ml NDC: 77302-001-02
100 ml NDC: 77302-001-02
237 ml NDC: 77302-001-03

237 ml NDC: 77302-001-03
500 ml NDC: 77302-001-04
 500 ml NDC: 77302-001-04
500 ml NDC: 77302-001-04
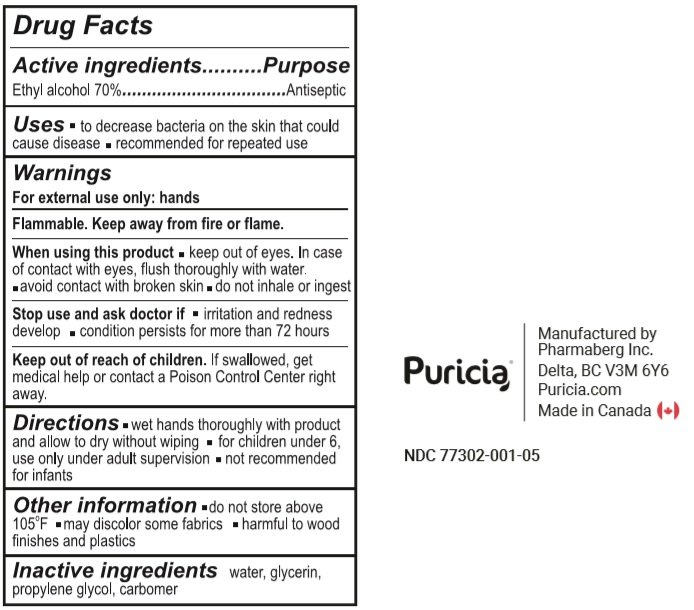
 1000 ml NDC: 77302-001-05
1000 ml NDC: 77302-001-05
 1000 ml NDC: 77302-001-05
1000 ml NDC: 77302-001-05
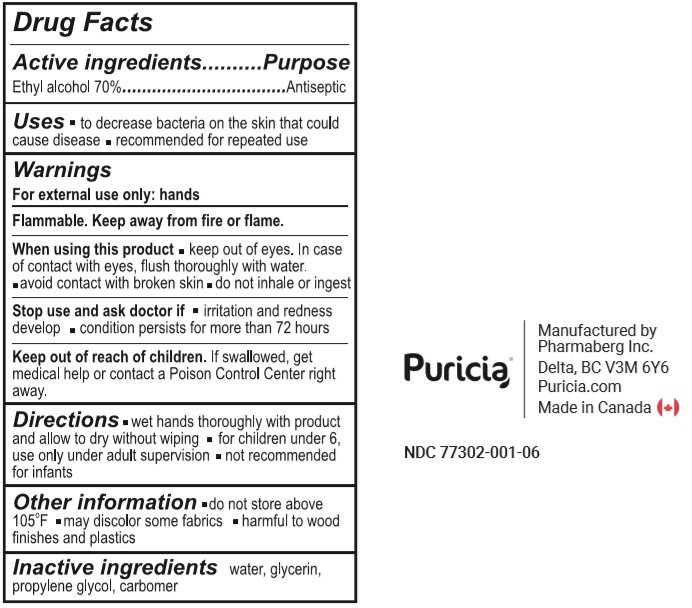
3.78 L NDC: 77302-001-06
 3.78 L NDC: 77302-001-06
3.78 L NDC: 77302-001-06
| PURICIA
ethyl alcohol 70% liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - PHARMABERG INC. (204355565) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
