BYD CARE Hand Sanitizer XD-A04
Dosage form: gel
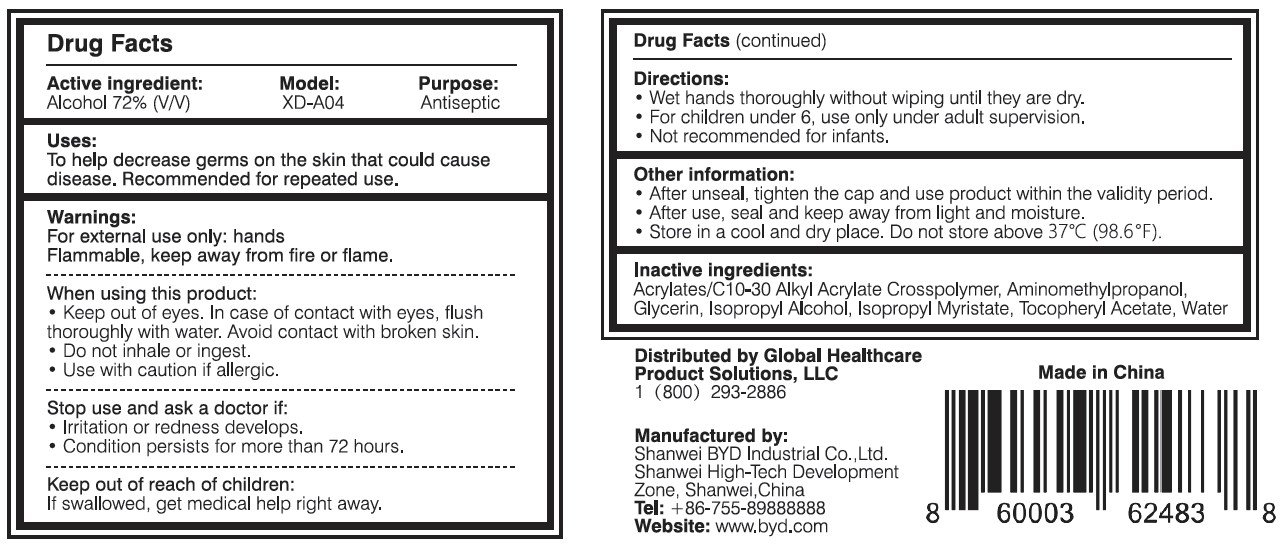
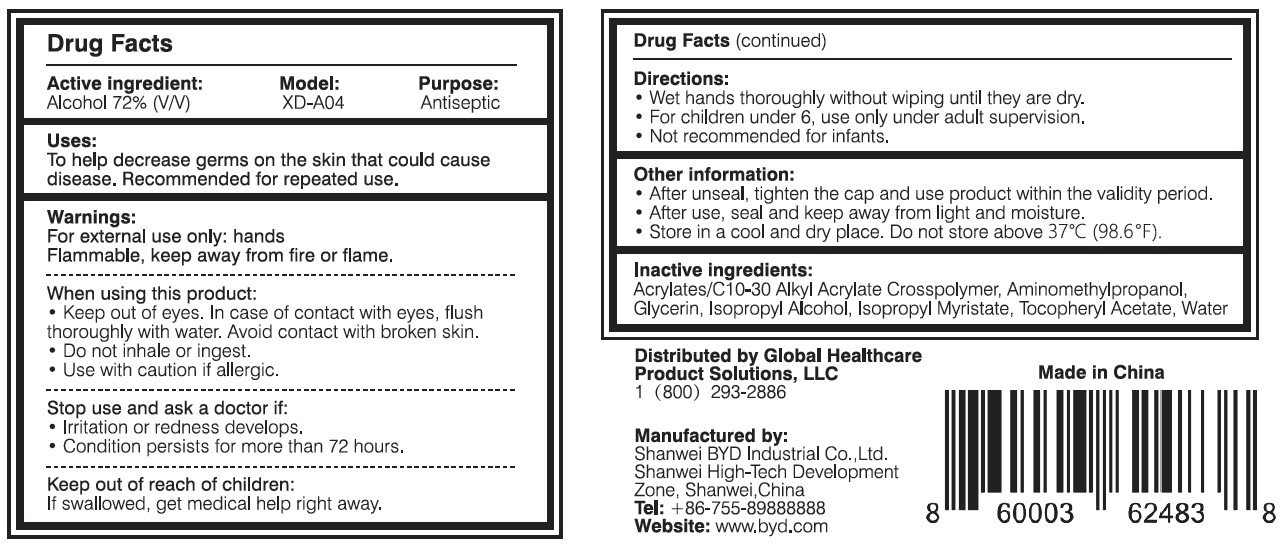
Ingredients: ALCOHOL 72mL in 100mL
Labeler: Shanwei BYD Industrial Co Ltd
NDC code: 73818-100
Medically reviewed by Drugs.com. Last updated on Apr 28, 2025.
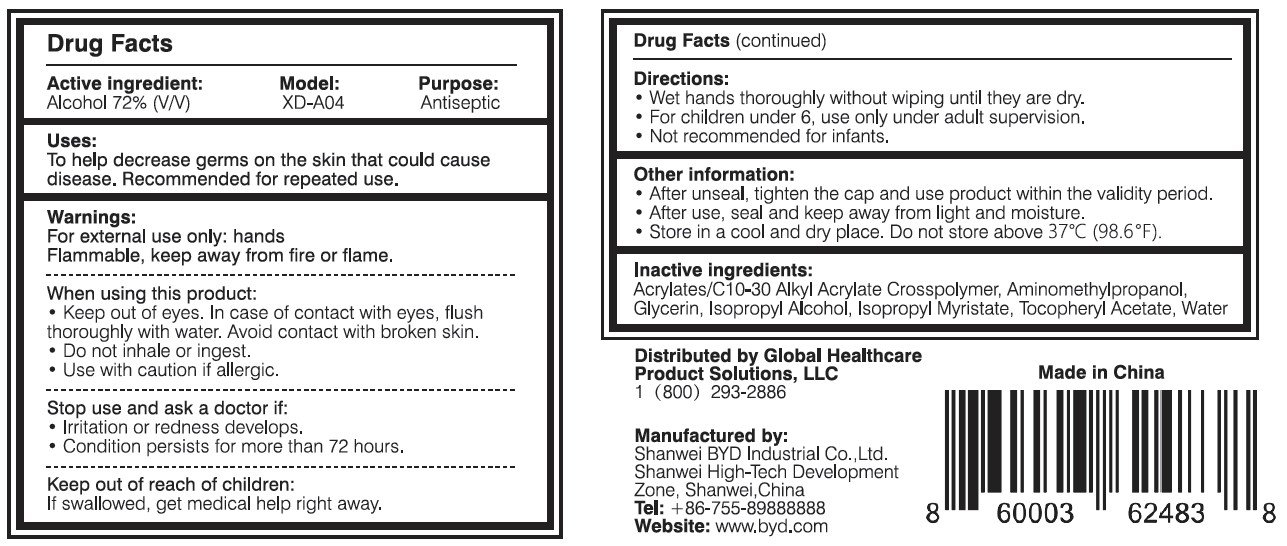
Distributed by Global Healthcare Product Solutions, LLC
1 (800) 293-2886
Manufactured by:
Shanwei BYD Industrial Co., Ltd.
Shanwei High-Tech Development Zone, Shanwei, China
Tel: +86-755-89888888
When using this product:
- Keep out of eyes. In case of contact with eyes, flush thoroughly with water. Avoid contact with broken skin.
- Do not inhale or ingest.
- Use with caution if allergic.
When using this product:
- Keep out of eyes. In case of contact with eyes, flush thoroughly with water. Avoid contact with broken skin.
- Do not inhale or ingest.
- Use with caution if allergic.
Stop use and ask a doctor if:
- Irritation or redness develops.
- Condition persists for more than 72 hours.
Keep out of reach of children:
If swallowed, get medical help or contact a Poison Control Center right away.
Directions:
- Wet hands thoroughly without wiping until they are dry.
- For children under 6, use only under adult supervision.
- Not recommended for infants.
Other information:
- After unseal, tighten the cap and use product within the validity period.
- After use, seal and keep away from light and moisture.
- Store in a cool and dry place. Do not store above 37 oC (98.6 oF).
| BYD CARE HAND SANITIZER XD-A04
alcohol gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Shanwei BYD Industrial Co Ltd (552102846) |
| Registrant - Shanwei BYD Industrial Co Ltd (552102846) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Shanwei BYD Industrial Co Ltd | 552102846 | manufacture(73818-100), label(73818-100), pack(73818-100) | |
Revised: 05/2020
Document Id: a5272f15-f6aa-7b07-e053-2995a90a186a
Set id: a5272e81-49a1-77e0-e053-2995a90aedb9
Version: 1
Effective Time: 20200508
Shanwei BYD Industrial Co Ltd
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.