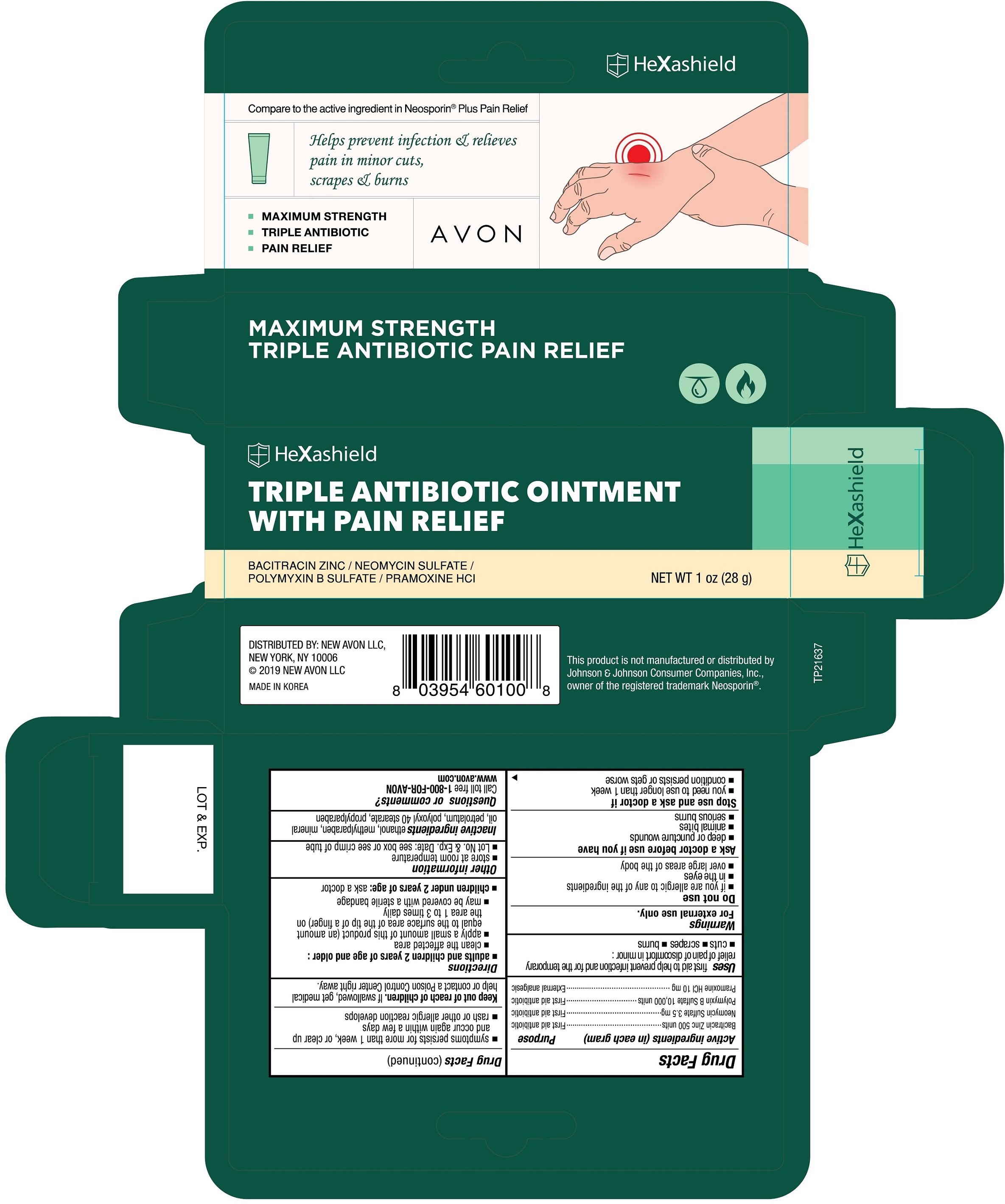
AVON HeXashield Triple Antibiotic with Pain Relief
Dosage form: ointment
Ingredients: POLYMYXIN B SULFATE 10000[USP'U] in 1g, NEOMYCIN SULFATE 3.5mg in 1g, BACITRACIN ZINC 500[USP'U] in 1g, PRAMOXINE HYDROCHLORIDE 10mg in 1g
Labeler: Tai Guk Pharm. Co., Ltd.
NDC code: 43136-100
Medically reviewed by Drugs.com. Last updated on Jul 24, 2025.
Bacitracin Zinc 500 units
Neomycin Sulfate 3.5 mg
Polymyxin B Sulfate 10,000 units
Pramoxine HCl 10 mg
First aid antibiotic
External analgesic
first aid to help prevent infection and for temporary relief of pain or discomfort in minor:
- cuts
- scrapes
- burns
For external use only.
- if you are allergic to any of the ingredients
- in the eyes
- over large areas of the body
- deep or puncture wounds
- animal bites
- serious burns
- you need to use longer than 1 week
- condition persists or gets worse
- symptoms persist for more than 1 week, or clear up and occur again within a few days
- rash or other allergic reaction develops
If swallowed, get medical help or contact a Poison Control Center right away.
- adults and children 2 years of age or older :
- clean the affected area
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: ask a doctor
- store at room temperature
- Lot No. & Exp. Date: see box or see crimp of tube
ethanol, methylparaben, mineral oil, petrolatum, polyoxyl 40 stearate, propylparaben
Call toll free 1-800-FOR-AVON
www.avon.com
Compare to the active ingredient in Neosporin ® Plus Pain Relief
Helps prevent infection & relieves pain in minor cuts, scrapes & burns
- MAXIMUM STRENGTH
- TRIPLE ANTIBIOTIC
- PAIN RELIEF
AVON
HeXashield
TRIPLE ANTIBIOTIC OINTMENT WITH PAIN RELIEF
BACITRACIN ZINC / NEOMYCIN SULFATE / POLYMYXIN B SULFATE / PRAMOXINE HCl
NET WT 1 oz (28g)

| AVON HEXASHIELD TRIPLE ANTIBIOTIC WITH PAIN RELIEF
bacitracin zinc, neomycin sulfate, polymyxin b sulfate, pramoxine hcl ointment |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Tai Guk Pharm. Co., Ltd. (689060246) |
| Registrant - New Avon LLC (080143520) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Tai Guk Pharma. Co., Ltd. | 689060246 | manufacture(43136-100) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
