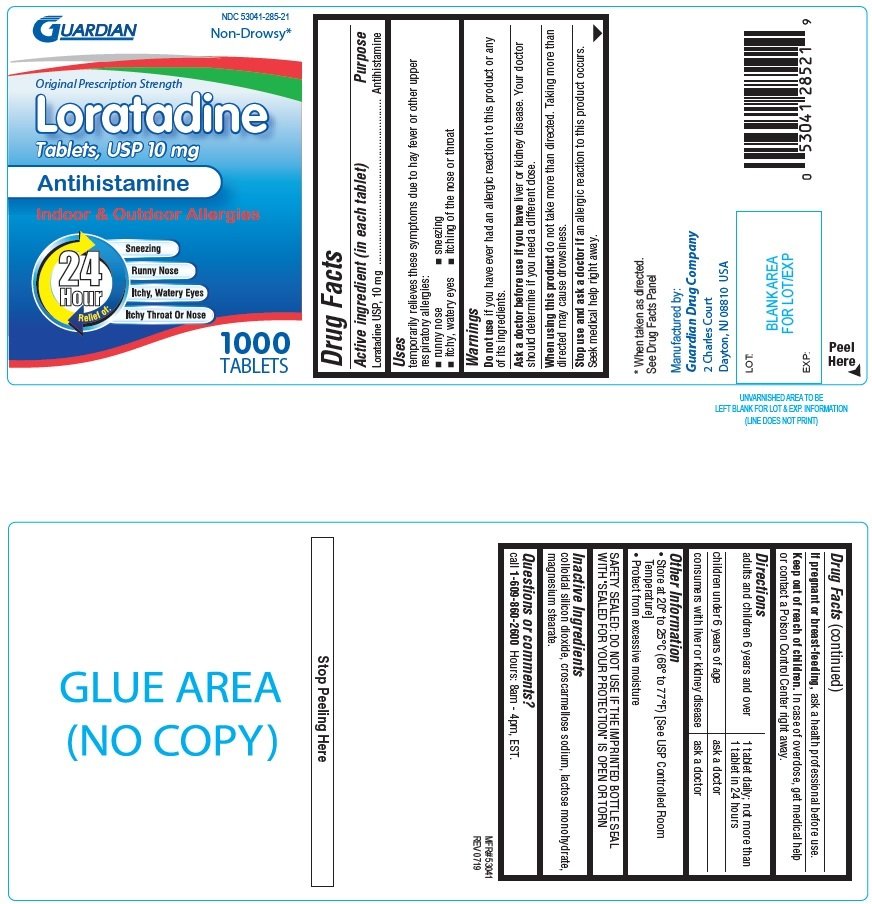
Guardian Loratadine
Dosage form: tablet
Ingredients: LORATADINE 10mg
Labeler: Guardian Drug Company
NDC code: 53041-285
Medically reviewed by Drugs.com. Last updated on Jul 14, 2025.
Loratadine USP, 10mg
Antihistamine
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
.
if you have ever had an allergic reaction to this product or any of its ingredients.
liver or kidney disease. Your doctor should determine if you need a different dose.
do not take more than directed. Taking more than directed may cause drowsiness.
an allergic reaction to this product occurs. Seek medical help right away.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
| adults and children 6 years and over | 1 tablet daily; not more than 1 tablet in 24 hours |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
- Store at 20o to 25oC (68o to 77oF) [See USP Controlled Room Temperature]
- Protect from excessive moisture
SAFETY SEALED: DO NOT USE IF THE IMPRINTED BOTTLE SEAL WITH "SEALED FOR YOUR PROTECTION" IS OPEN OR TORN
colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate
call 1-609-860-2600 Hours: 8am-4pm, EST.
| GUARDIAN LORATADINE
loratadine tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Guardian Drug Company (119210276) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Guardian Drug Company | 119210276 | MANUFACTURE(53041-285) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.