Skyn Endurance Delay For Men
Dosage form: liquid
Ingredients: LIDOCAINE 125mg in 1mL
Labeler: Sxwell USA, LLC
NDC code: 73115-000
Medically reviewed by Drugs.com. Last updated on Jun 30, 2025.
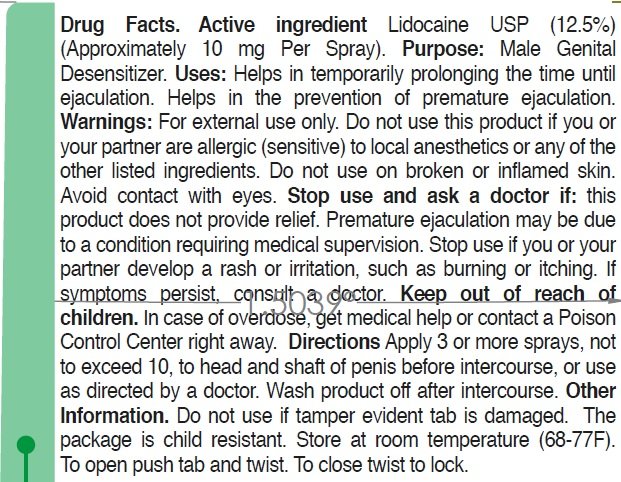
Lidocaine USP (12.5%) (Approximately 10 mg Per Spray)
Male Genital Desensitizer
• Helps in temporarily prolonging the time until ejaculation.
• Helps in the prevention of premature ejaculation.
For external use only
this product if you or your partner are allergic (sensitive) to local anesthetics or any of the other listed ingredients. Do not use on broken or inflamed skin.
avoid contact with eyes.
• This product, used as directed, does not provide relief, discontinue use and consult a doctor. Premature ejaculation may be due to a condition requiring medical supervision.
• You or your partner develop a rash or irritation, such as burning or itching, discontinue use. If symptoms persist, consult a doctor.
In case of overdose, get medical help or contact a Poison Control Center right away.
• Apply 3 or more sprays, not to exceed 10, to head and shaft of penis before intercourse, or use as directed by a doctor.
• Wash product off after intercourse.
• Do not use if temper evident tab is damaged.
• The package is child resistant.
• Store at room temperature (68-77F).
Isopropyl Myristate, L-Arginine, Peppermint Oil, Phenoxyethanol.


| SKYN ENDURANCE DELAY FOR MEN
lidocaine liquid |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - Sxwell USA, LLC (080650902) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
