Haws Eyewash Additive
Dosage form: solution, concentrate
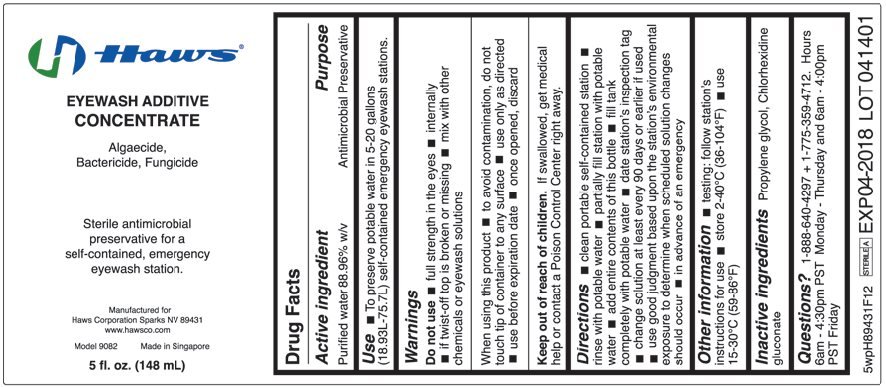
Ingredients: WATER 889.6g in 1000mL
Labeler: Haws Corporation
NDC code: 66051-210
Medically reviewed by Drugs.com. Last updated on Jan 13, 2025.
Purified water 88.96% w/v
Antimicrobial Preservative
To preserve potable water in 5-20 gallons (18.93L-75.7L) self-contained emergency eyewash stations.
Do not use
- •
- full strength in the eyes
- •
- internally
- •
- if twist-off top is broken or missing
- •
- mix with other chemicals or eyewash solutions
When using this product
- •
- to avoid contamination, do not touch tip of container to any surface
- •
- use only as directed
- •
- use before expiration date
- •
- once opened, discard
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- •
- clean portable self-contained station
- •
- rinse with potable water
- •
- partially fill station with potable water
- •
- add entire contents of this bottle
- •
- fill tank completely with potable water
- •
- date station’s inspection tag
- •
- change solution at least every 90 days or earlier if used
- •
- use good judgment based upon the station’s environmental exposure to determine when scheduled solution changes should occur
- •
- in advance of an emergency
- •
- testing: follow station’s instructions for use
- •
- store 2-40°C (36-104°F)
- •
- use 15-30°C (59-86°F)
Propylene glycol, Chlorhexidine gluconate
1-888-640-4297 + 1-775-359-4712. Hours 6am – 4:30pm PST Monday – Thursday and 6am – 4:00pm PST Friday
Haws
EYEWASH ADDITIVE CONCENTRATE
Algaecide, Bactericide, Fungicide
Sterile antimicrobial preservative for a self-contained, emergency eyewash station.
Manufactured for
Haws Corporation Sparks NV 89431
www.hawsco.com
Model 9082
Made in Singapore
5 fl. oz. (148 mL)
| HAWS EYEWASH ADDITIVE
purified water solution, concentrate |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - Haws Corporation (009124785) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
