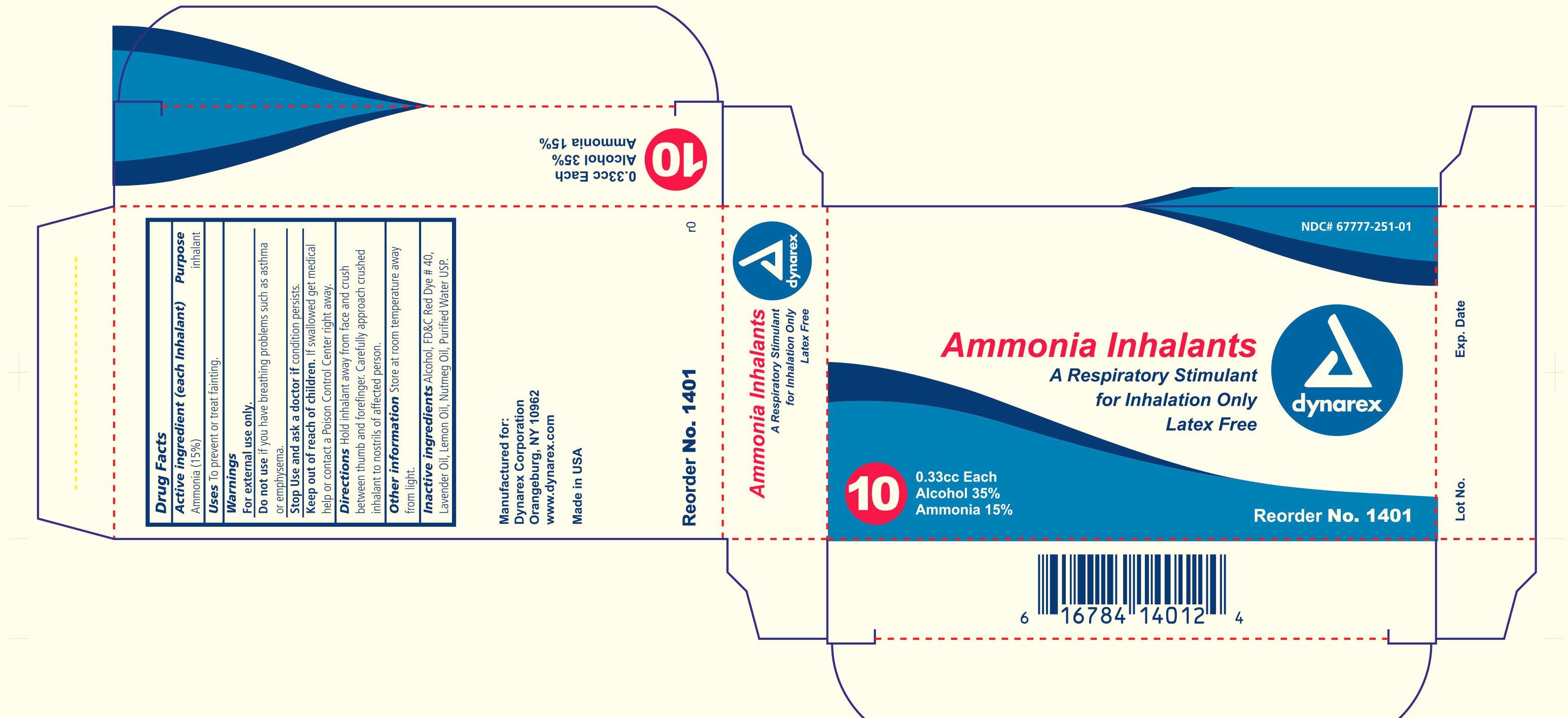
Ammonia Inhalants by Dynarex Corporation
Dosage form: inhalant
Ingredients: AMMONIA 0.05g in 0.33mL
Labeler: Dynarex Corporation
NDC code: 67777-251
Medically reviewed by Drugs.com. Last updated on Mar 10, 2025.
active ingredient each inhalant purpose ammonia 15% inhalant
Uses to prevent or treating fainting
warnings for external use only
do not use if you have breathing problems such as asthma or emphysema
stop use and ask a doctor if condition persists
If swallowed get medical help or contact a Poison Control Center right away
Hold inhalant away from face and crush between thumb and forefinger. carefully approach crushed inhalant to nostrils of affected person.
Store at room temperature away from light
alcohol fdc red dye 40 lavender oil, lemon oil, nutmeg oil, purified water usp
to prevent or treat fainting
for external use only
| AMMONIA INHALANTS
ammonia inhalants inhalant |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - Dynarex Corporation (008124539) |
| Registrant - Dynarex Corporation (008124529) |
See all Ammonia Inhalants brands
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.