Womens Mitchum Clinical Antiperspirant Deodorant Soft Solid Powder Fresh
Dosage form: cream
Ingredients: ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY 0.20g in 1g
Labeler: Revlon Consumer Products Corp
NDC code: 10967-595
Medically reviewed by Drugs.com. Last updated on Feb 7, 2025.
Active Ingredient
Aluminum zirconium tetrachlorohydrex gly 20%
Antiperspirant
- Reduces underarm wetness
For external use only.
Do not use on broken skin
Ask a doctor before use if you have kidney disease
Stop use if rash or irriation occurs
If swallowed, get medical help or contact a Poison Control Center right away.
Apply to underarms only.
cyclopentasiloxane, tribehenin, dimethicone, parfum (fragrance), petrolatum, trisiloxane, c18-36 acid triglyceride, hydrogen peroxide, tocopheryl acetate, aloe barbadensis leaf extract, sodium starch octenylsuccinate, aqua((water) eau), silica dimethicone silylate, silica, benzyl salicylate, linalool, limonene, hexyl cinnamal, coumarin, citronellol, geraniol, cinnamyl alcohol, citral
1-888-8-MITCHUM
Primary Principal Display Panel - 1.6 oz.
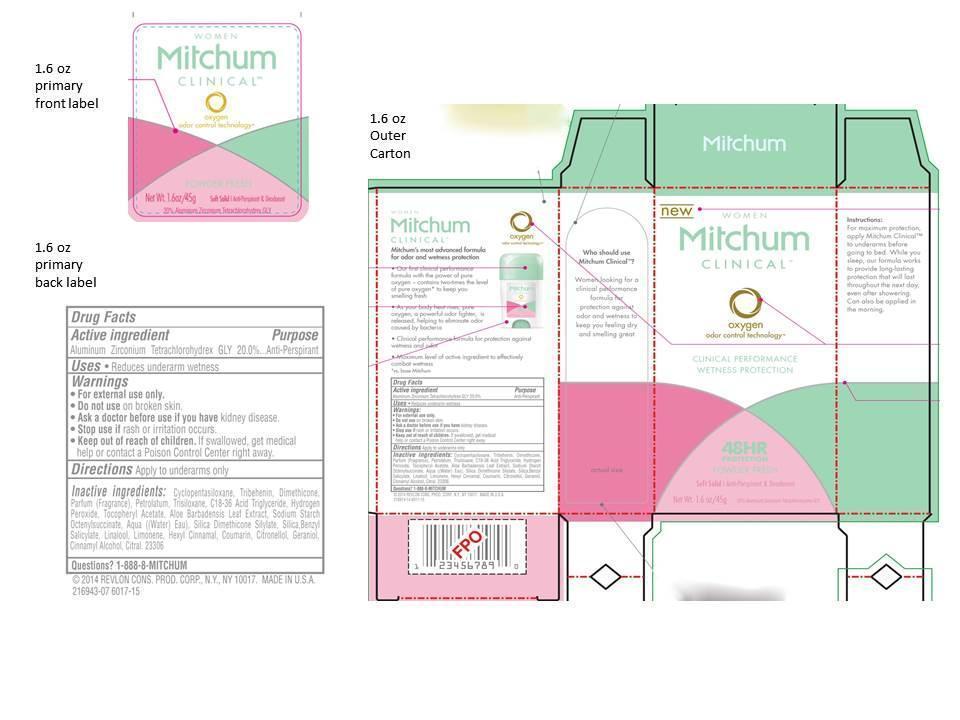
| WOMENS MITCHUM CLINICAL ANTIPERSPIRANT DEODORANT
SOFT SOLID POWDER FRESH
aluminum zirconium tetrachlorohydrex gly cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Revlon Consumer Products Corp (788820165) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Dimensional Mechandising, Inc. | 076693183 | manufacture(10967-595) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
