Rugby Barrier Cream
Dosage form: cream
Ingredients: ZINC OXIDE 200mg in 1g, MENTHOL 4.5mg in 1g
Labeler: Rugby Laboratories
NDC code: 0536-1204
Medically reviewed by Drugs.com. Last updated on Apr 23, 2025.
Drug Facts
Menthol 0.45%
Zinc Oxide 20%
External Analgesic
Astringent
Multipurpose moisture barrier that aids in protecting irritated skin conditions in the perineum, buttocks, lower abdomen and inner thigh due to moisture, occlusion, chafing or continued contact with urine or feces.
Helps relieve the local itching, discomfort, pain, burning and irritation associated with anorectal disorders and hemorroids.May provide a cooling sensation.
•in case of bleeding
•if conditions worsenor deos not improve within 7 days
<Do not exceed recommended daily dosage unless directed by a doctor. Do not put product into rectum by using fingers or any mechanical device or applicatorAllergy Alert:Certain persons can develop allergic reactions to this product. If the symptoms develop or increase,discontinue use and consult a doctor. >
If swallowed, get medical help or contact a Poison Control Center right away.
Store at controlled room temperature 20° - 25°C (68° - 77°F)
Calamine, Codliver Oil, Disodium EDTA, Lanolin, Methylparaben, Microcrystalline Wax, Mineral Oil, Petrolatum, Propylparaben, Sodium Borate, Sorbitan Sequioleate, Talc, Water (Purified)
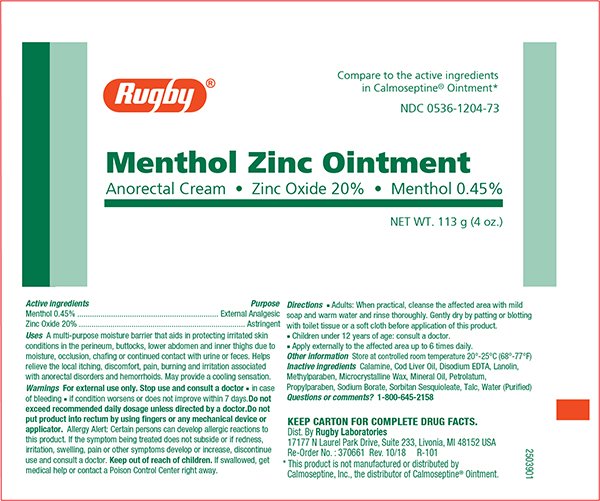
NDC 0536-1204-73
Rugby
Menthol Zinc Ointment
Anorectal Cream
0.45% Menthol -External Analgesic
20% Zinc Oxide -Astringent
• Protect and soothes irritated or chafed skin associated with incontinence
• Temporary relief of pain & itching
NET WT. 4 oz. (113g)



NDC 0536-1204-73
Rugby
Menthol Zinc Ointment
Anorectal Cream
0.45% Menthol
20% Zinc Oxide
Net Wt. 4 oz 113 g

| RUGBY BARRIER CREAM
barrier cream cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Rugby Laboratories (079246066) |
| Registrant - Sheffield Pharmaceuticals, LLC (151177797) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sheffield Pharmaceuticals, LLC | 151177797 | MANUFACTURE(0536-1204) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
