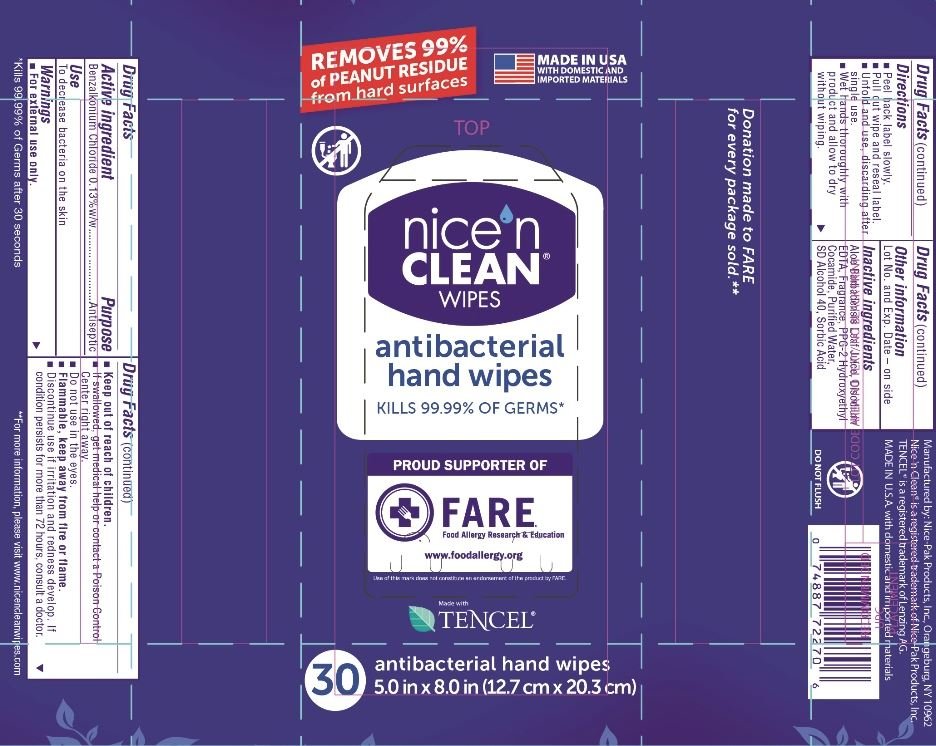
Nice N Clean Antimicrobial Hand Wipes
Dosage form: swab
Ingredients: BENZALKONIUM CHLORIDE 1.3mg in 1mL
Labeler: Nice-Pak Products, Inc.
NDC code: 44385-7013
Medically reviewed by Drugs.com. Last updated on Jun 16, 2025.
Active ingredient
Benzalkonium Chloride 0.13%w/w
Purpose
Antiseptic
Use
To decrease bacteria on the skin
Warnings
For external use only.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Do not use in the eyes.
Flammable, keep away from fire or flame.
Discontinue use if irritation and redness develop.
If condition persists for more than 72 hours, consult a doctor.
Directions
Peal back label slowly.
Pull out wipe and reseal label.
Unfold and use, discarding after single use.
Wet hands thoroughly with product and allow to dry without wiping.
Other information
Lot No. and Exp. Date - on side
inactive ingredients
Aloe Barbadensis Leaf Juice, Disodium EDTA, Fragrance, PPG-2 Hydroxyethyl Cocamide, Purified Water, SD Alcohol 40, Sorbic Acid
REMOVES 99%
of PEANUT RESIDUE
from hard surfaces
(Flag Logo)
MADE IN USA
WITH DOMESTIC AND
IMPORTED MATERIALS
(Do not dispose in toilet Logo)
NICE N
CLEAN
WIPES
antibacterial
hand wipes
KILLS 99.99% of GERMS*
PROUD SUPPORTER OF
(Logo) FARE
Food Allergy Research and Education
www.foodallergy.org
Made with
(Leaf Logo) TENCEL
30 antibacterial hand wipes
5.0 in x 8.0 in (12.7 cm x 20.3 cm)


| NICE N CLEAN ANTIMICROBIAL HAND WIPES
antibacterial hand wipes swab |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Nice-Pak Products, Inc. (003778198) |
| Registrant - Nice-Pak Products, Inc. (003778198) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Nice-Pak Products, Inc. | 067900167 | manufacture(44385-7013) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Tongling Jieya Biological Technology Stock Co., Ltd. | 529736361 | manufacture(44385-7013) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Professional Disposables International, Inc. | 800777117 | manufacture(44385-7013) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
