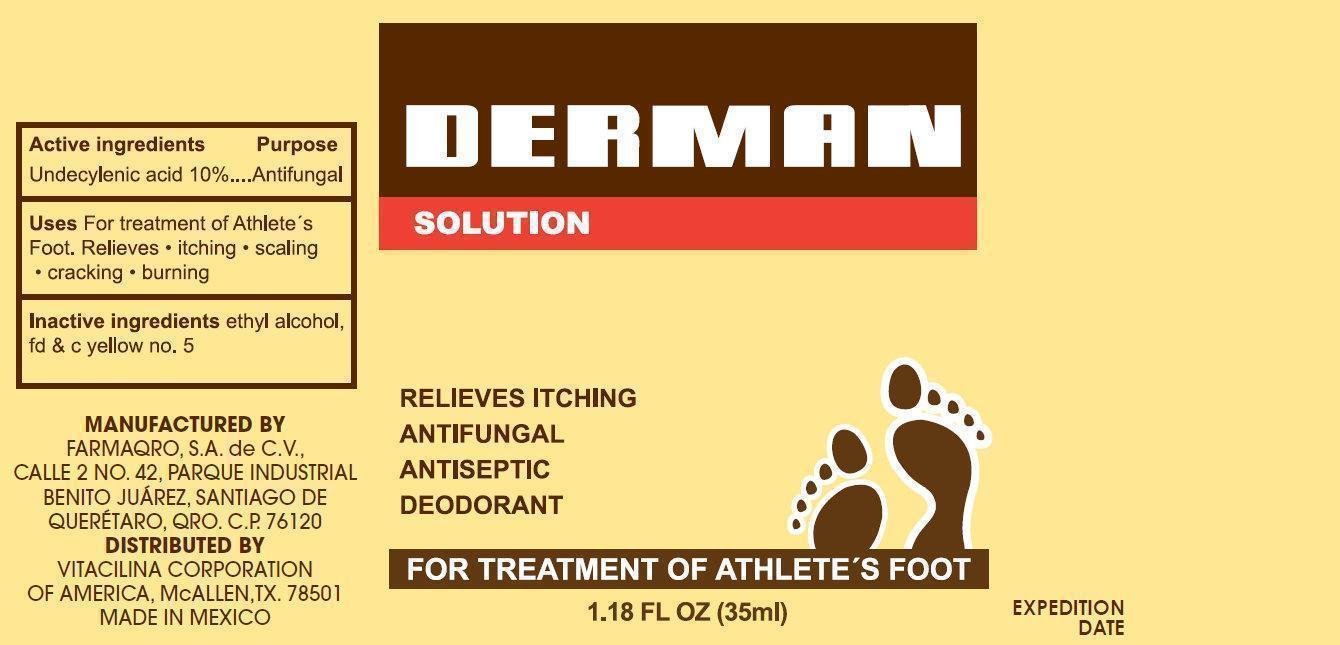
DERMAN ANTIFUNGAL
Dosage form: spray
Ingredients: UNDECYLENIC ACID 100mg in 1mL
Labeler: Compania Internacional de Comercio, S.A. de C.V.
NDC code: 54312-325
Medically reviewed by Drugs.com. Last updated on Nov 4, 2024.
Undecylenic acid 10%
Antifungal
For treatment of Athlete's Foot Relieves • itching • scaling • cracking • burning
For external use only
Avoid contact with the eyes
on children under 2 years of age unless directed by a doctor
occurs or if there is no improvement within 4 weeks, discontinue use and consult a doctor.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Wash the affected area and dry thoroughly. Apply a few drops of the product over affected area twice daily (morning and night) or as directed by a doctor. Supervise children in the use of this product. Pay special attention to spaces between the toes; wear well-fitting, ventilated shoes, and change shoes and socks at least once daily. Use daily for 4 weeks. If condition persists longer, consult a doctor. This product is not effective on the scalp and nails.
ethyl alcohol, fdandc yellow no. 5

| DERMAN ANTIFUNGAL
undecylenic acid spray |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - Compania Internacional de Comercio, S.A. de C.V. (815039995) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Farmaqro, S.A. de C.V. | 588161468 | manufacture(54312-325) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
