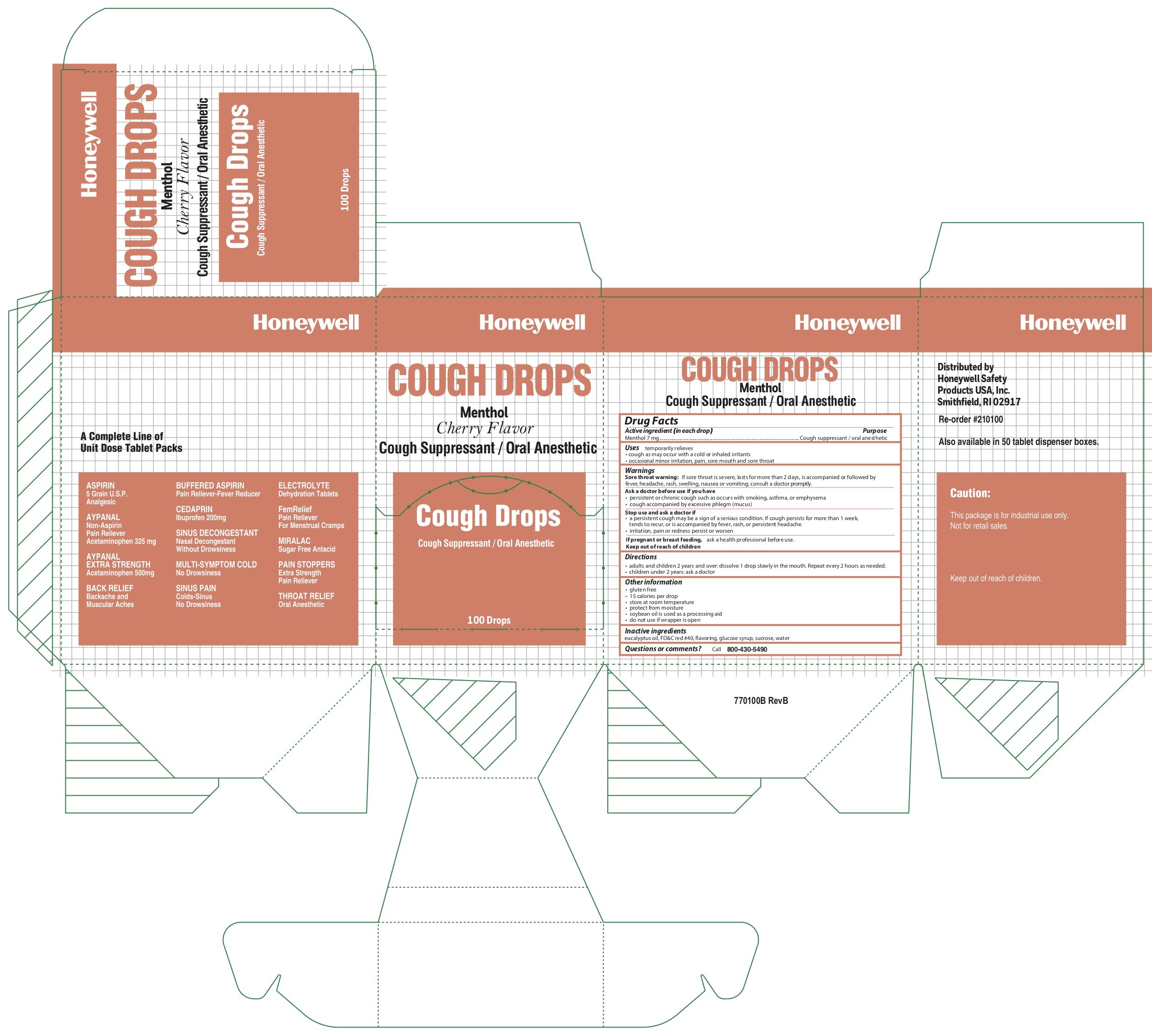
Cough Drop
Dosage form: lozenge
Ingredients: MENTHOL 7mg
Labeler: Honeywell Safety Products USA, Inc
NDC code: 0498-1120
Medically reviewed by Drugs.com. Last updated on Jan 9, 2025.
Active ingredient (in each drop)
Menthol 7 mg
Purpose
Cough suppressant /oral anesthetic
Uses
temporarily relieves
- cough as may occur with a cold or inhaled irritants
- occasional minor irritation, pain, sore mouth and sore throat
Warnings
Sore throat warning: if sore throat is severe, lasts for more than 2 days, is accompanied or followed by a fever, headache, rash, swelling, nausea or vomiting, consult a doctor promptly
Ask a doctor before use if you have:
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- cough accompanied by excessive phlegm (mucus)
Stop use and ask a doctor if
- a persistant cough may be a sign of a serious condition. If cough persists for more than 1 week, tends to recur, or is accompaniedby fever, rash, or persistant headache.
- irritation, pain, or redness persist or worsen
If pregnant or breast feeding:
- ask a health professional before use
Keep this and all drugs out of the reach of children
Enter section text here
Directions
- adults and children 2 years of age and over
- dissolve 1 drop slowly in the mouth. Repeat every 2 hours as needed
- children under 2 years of age: ask a doctor
Other information
- gluten free
- 15 calories per cough drop
- store at room temperature
- protect from moisture
- soybean oil used as a processing aid
- do not use if wrapper is open
Inactive ingredients
eucalyptus oil, FD &C red #40, flavoring, glucose syrup, sucrose, water
Questions or comments?
call 800-430-5490
| COUGH DROP
menthol lozenge |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| NORTH COUGH DROP
menthol lozenge |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| NORTH COUGH DROP
menthol lozenge |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Honeywell Safety Products USA, Inc (079287321) |
| Registrant - Honeywell Safety Products USA, Inc (079287321) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Honeywell Safety Products USA, Inc | 079287321 | pack(0498-0119, 0498-1119, 0498-1120) | |
Revised: 01/2020
Document Id: 9c87d588-75b6-152b-e053-2995a90a32fc
Set id: 87fd9e18-8681-47a4-8614-239b8b55d69f
Version: 11
Effective Time: 20200119
Honeywell Safety Products USA, Inc
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.