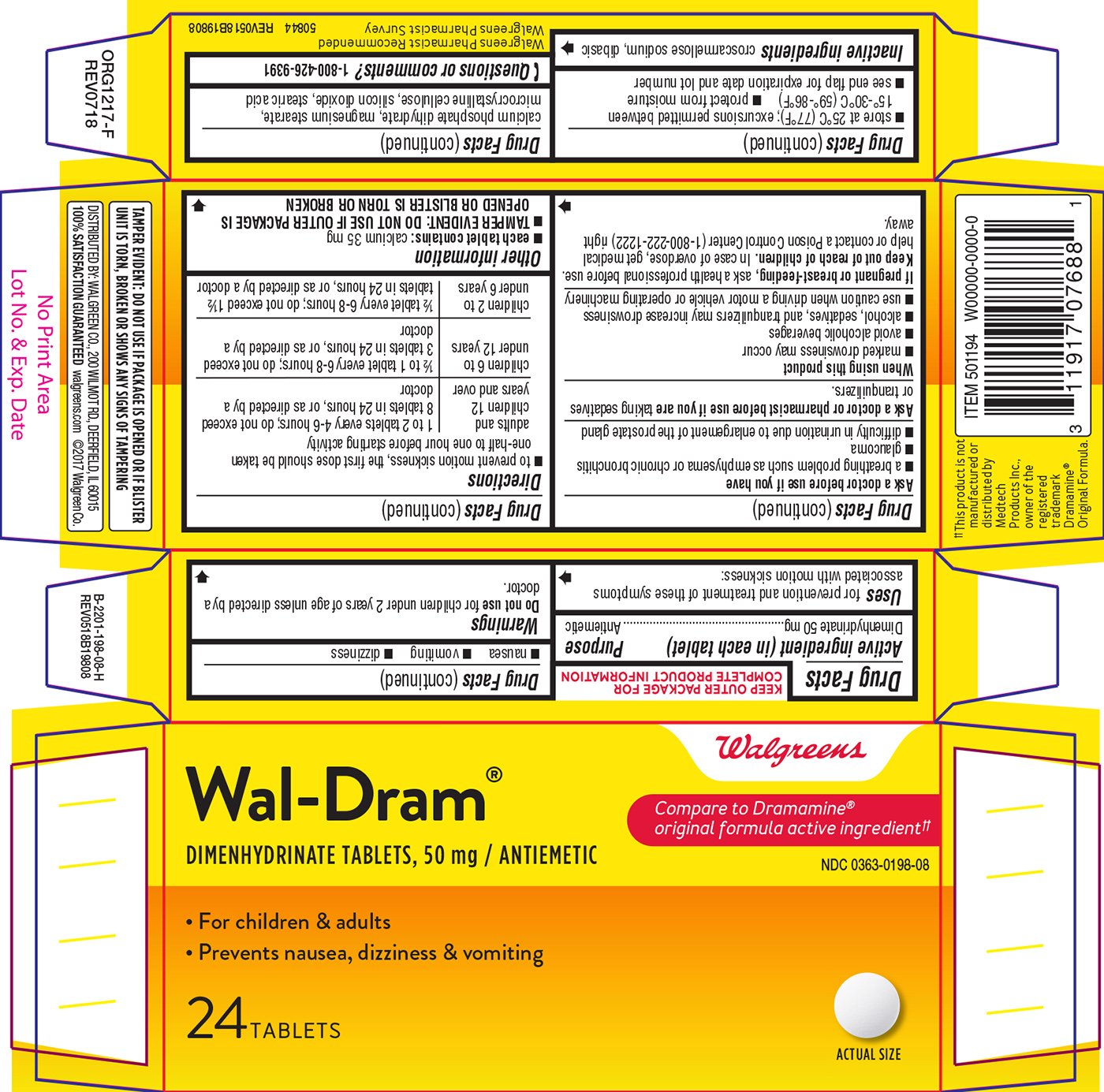
Wal-Dram
Dosage form: tablet
Ingredients: DIMENHYDRINATE 50mg
Labeler: Walgreen Company
NDC code: 0363-0198
Medically reviewed by Drugs.com. Last updated on Jul 24, 2025.
Dimenhydrinate 50 mg
Antiemetic
for prevention and treatment of these symptoms associated with motion sickness:
- nausea
- vomiting
- dizziness
for children under 2 years of age unless directed by a doctor.
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
taking sedatives or tranquilizers.
- marked drowsiness may occur
- avoid alcoholic beverages
- alcohol, sedatives, and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
- to prevent motion sickness, the first dose should be taken one-half to one hour before starting activity
| adults and children 12 years and over | 1 to 2 tablets every 4-6 hours; do not exceed 8 tablets in 24 hours, or as directed by a doctor |
| children 6 to under 12 years | 1/2 to 1 tablet every 6-8 hours; do not exceed 3 tablets in 24 hours, or as directed by a doctor |
| children 2 to under 6 years | 1/2 tablet every 6-8 hours; do not exceed 1 1/2 tablets in 24 hours, or as directed by a doctor |
-
each tablet contains: calcium 35 mg
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- protect from moisture
- see end flap for expiration date and lot number
croscarmellose sodium, dibasic calcium phosphate dihydrate, magnesium stearate, microcrystalline cellulose, silicon dioxide, stearic acid
1-800-426-9391
Walgreens
Compare to Dramamine® original formula active ingredient††
NDC 0363-0198-08
Wal-Dram®
DIMENHYDRINATE TABLETS, 50 mg / ANTIEMETIC
• For children & adults
• Prevents nausea, dizziness & vomiting
24 TABLETS
ACTUAL SIZE
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
DISTRIBUTED BY: WALGREEN CO., 200 WILMOT RD., DEERFIELD, IL 60015
100% SATISFACTION GUARANTEED walgreens.com ©2017 Walgreen Co.
††This product is not manufactured or distributed by Medtech Products Inc., owner of the registered trademark Dramamine® Original Formula.
Walgreens Pharmacist Recommended
Walgreens Pharmacist Survey
50844 REV0518B19808
ORG1217-F
REV0718
Walgreens 44-198
| WAL-DRAM
dimenhydrinate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Walgreen Company (008965063) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867894 | MANUFACTURE(0363-0198) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 038154464 | PACK(0363-0198) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867837 | PACK(0363-0198) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 967626305 | PACK(0363-0198) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 868734088 | PACK(0363-0198) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
