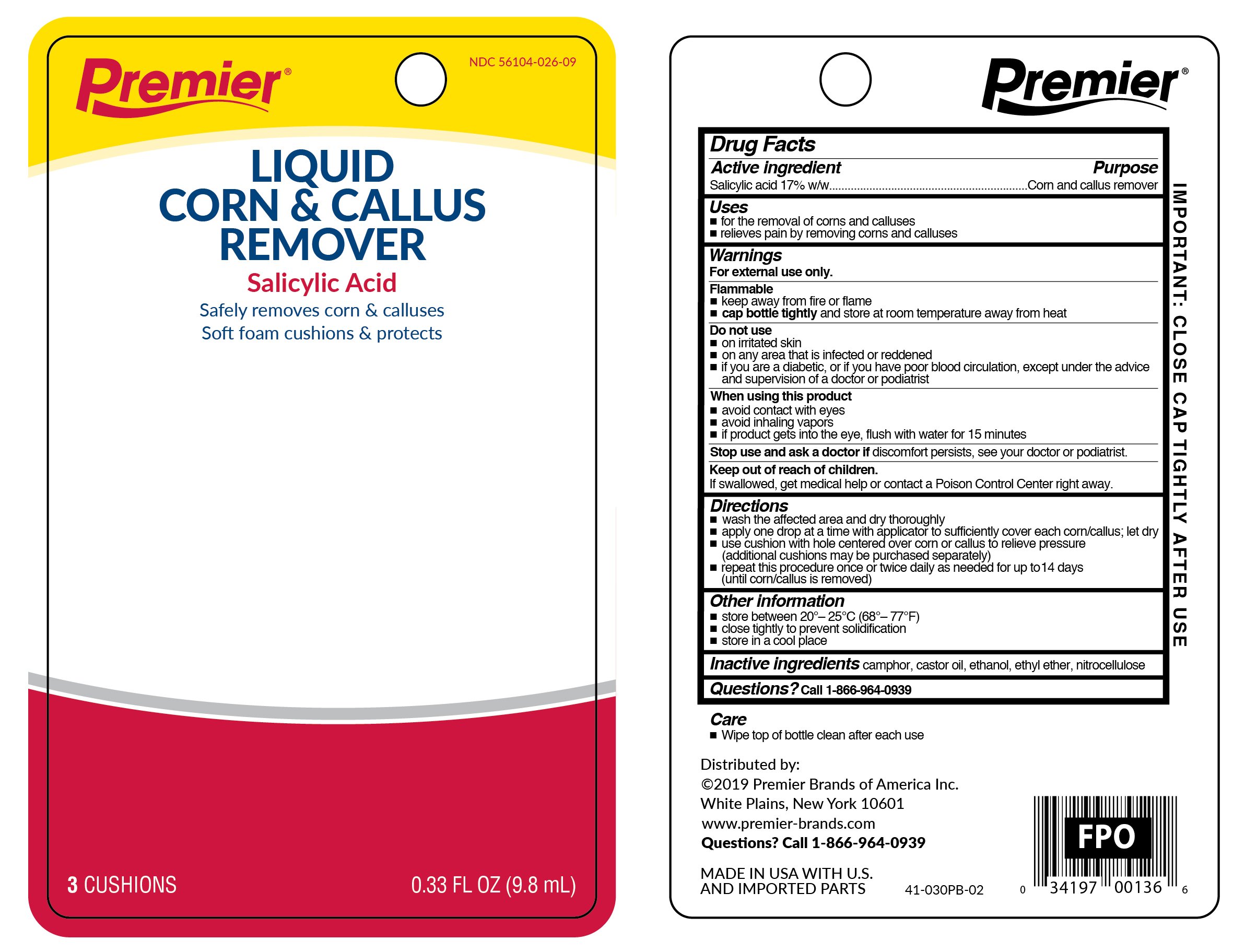
Cornex
Dosage form: liquid
Ingredients: SALICYLIC ACID 0.17g in 1g
Labeler: Premier Brands of America Inc.
NDC code: 56104-009
Medically reviewed by Drugs.com. Last updated on Sep 17, 2024.
Active ingredient
Salicylic acid 17%w/w
Purpose
Corn and callus remover
Uses
- for the removal of corns and calluses
- relieves pain by removing corns and calluses
Warnings
For external use only.
Flammable
- keep away from fire or flame
- cap bottle tightly and store at room temperature away heat
Do not use
- on irritated skin
- on any area that is infected or reddened
- if you are a diabetic, or if you have poort blood circulation, excpet under the advice and supervision of a doctor or podiatrist
When using this product
- avoid contact with eyes
- avoid inhaling vapors
- if product gets into the eyes, flush with water for 15 minutes
Stop and ask a doctor if
discomfort persists, see your doctor or podiatrist
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wash affected area and dry thoroughly
- apply one drop at a time with applicator, to sufficiently cover each corn/callus; let dry
- use cushions with hole centered over corn or callus to relieve pressure (additional cushions may be purchased separately)
- repeat this procedure once or twice daily as needed for up to 14 days (until corn/callus is removed)
Other information
- store between 20°C to 30°C (68°F to 86°F)
- clsoe tightly to prevent solidification
- store in a cool place
Inactive ingredients
camphor, castro oil, ethanol, ethyl ether, nitrocellulose
Questions?
Call 1-866-964-0939
Principal Display Panel
Premier
Liquid Corn & Callus Remover
Salicylic Acid
Safely removes corn & calluses
Soft foam cushions and protects
3 CUSHIONS 0.33 FL OZ (9.8 mL)

| CORNEX
corn and callus liquid remover liquid |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - Premier Brands of America Inc. (080051232) |
Document Id: 938f3132-e355-a9bb-e053-2a95a90a44d9
Set id: 4f0b5582-80e6-432b-afd3-de251b6b2451
Version: 5
Premier Brands of America Inc.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
