Kroger Antifungal
Dosage form: cream
Ingredients: Terbinafine Hydrochloride 1g in 100g
Labeler: Kroger Company
NDC code: 30142-080
Medically reviewed by Drugs.com. Last updated on Jul 21, 2025.
Antifungal
Drug Facts
Terbinafine hydrochloride 1%
Antifungal
- cures most athlete's foot (tinea pedis)
- cures most jock itch (tinea cruris) and ringworm (tinea corporis)
- relieves itching, burning, cracking and scaling which accompany these conditions
For external use only
- on nails or scalp
- in or near the mouth or eyes
- for vaginal yeast infections
When using this product do not get into eyes. If eye contact occurs, rinse thoroughly with water.
- too much irritation occurs or gets worse
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- adults and children 12 years and over:
- use the tip of the cap to break the seal and open the tube
- wash the affected skin with soap and water and dry completely before applying
-
for athlete's foot wear well-fitting, ventilated shoes. Change shoes and socks at least once daily.
- between the toes only: apply twice a day (morning and night) for 1 week or as directed by a doctor
- on the bottom or sides of the foot: apply twice a day (morning and night) for 2 weeks or as directed by a doctor
- for jock itch and ringworm: apply once a day (morning or night) for 1 week or as directed by a doctor
- wash hands after each use
- children under 12 years: ask a doctor

1 week between the toes
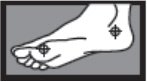
2 weeks on the bottom or sides of the foot
TAMPER EVIDENT: DO NOT USE IF THE SEAL ON THE TUBE IS PUNCTURED OR NOT VISIBLE.
- store at controlled room temperature 20° to 25°C (68° to 77°F)
- see carton or tube crimp for lot number and expiration date
benzyl alcohol, cetyl alcohol, cetyl palmitate, isopropyl myristate, polysorbate 60, purified water, sodium hydroxide, sorbitan monostearate, stearyl alcohol
call 1-800-632-6900
DISTRIBUTED BY THE KROGER CO.
CINCINNATI, OHIO 45202
Kroger®
Athlete's
Foot Cream
Terbinafine Hydrochloride Cream 1%
ANTIFUNGAL
Clinically Proven to Cure Most
Athlete's Foot Between the Toes
Relieves Itching
NET WT 0.5 OZ (15 g)

| KROGER
ANTIFUNGAL
terbinafine hydrochloride cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Kroger Company (006999528) |
| Registrant - Taro Pharmaceuticals U.S. A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Taro Pharmaceuticals Inc. | 206263295 | MANUFACTURE(30142-080) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
