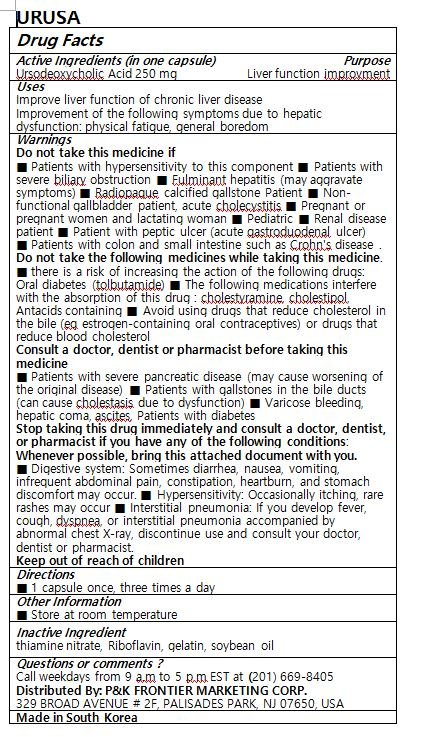
URUSA
Dosage form: capsule
Ingredients: URSODIOL 250mg
Labeler: OASIS TRADING
NDC code: 72689-0001
Medically reviewed by Drugs.com. Last updated on Nov 11, 2024.
Ursodeoxycholic Acid
Improve liver function of chronic liver disease
Improvement of the following symptoms due to hepatic dysfunction: physical fatigue, general boredom
Keep out of reach of children
1 capsule once, three times a day
Do not take this medicine if
■ Patients with hypersensitivity to this component ■ Patients with severe biliary obstruction ■ Fulminant hepatitis (may aggravate symptoms) ■ Radiopaque calcified gallstone Patient ■ Non-functional gallbladder patient, acute cholecystitis ■ Pregnant or pregnant women and lactating woman ■ Pediatric ■ Renal disease patient ■ Patient with peptic ulcer (acute gastroduodenal ulcer)
■ Patients with colon and small intestine such as Crohn's disease .
Do not take the following medicines while taking this medicine.
■ there is a risk of increasing the action of the following drugs: Oral diabetes (tolbutamide) ■ The following medications interfere with the absorption of this drug : cholestyramine, cholestipol, Antacids containing ■ Avoid using drugs that reduce cholesterol in the bile (eg estrogen-containing oral contraceptives) or drugs that reduce blood cholesterol
Consult a doctor, dentist or pharmacist before taking this medicine
■ Patients with severe pancreatic disease (may cause worsening of the original disease) ■ Patients with gallstones in the bile ducts (can cause cholestasis due to dysfunction) ■ Varicose bleeding, hepatic coma, ascites, Patients with diabetes
Stop taking this drug immediately and consult a doctor, dentist, or pharmacist if you have any of the following conditions: Whenever possible, bring this attached document with you.
■ Digestive system: Sometimes diarrhea, nausea, vomiting, infrequent abdominal pain, constipation, heartburn, and stomach discomfort may occur. ■ Hypersensitivity: Occasionally itching, rare rashes may occur ■ Interstitial pneumonia: If you develop fever, cough, dyspnea, or interstitial pneumonia accompanied by abnormal chest X-ray, discontinue use and consult your doctor, dentist or pharmacist.
thiamine nitrate, Riboflavin, gelatin, soybean oil
For oral use only
| URUSA
ursodeoxycholic acid capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - OASIS TRADING (689991468) |
| Registrant - OASIS TRADING (689991468) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| OASIS TRADING | 689991468 | manufacture(72689-0001) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
