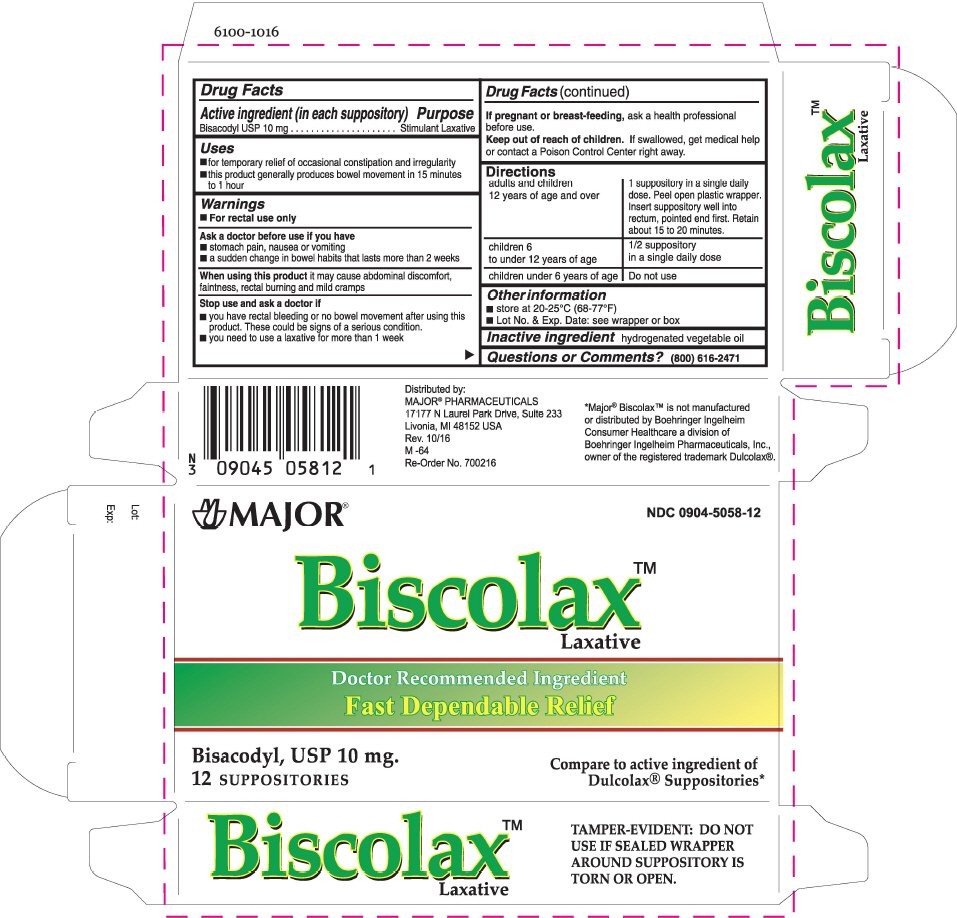
Biscolax Laxative
Dosage form: suppository
Ingredients: BISACODYL 10mg
Labeler: Major
NDC code: 0904-5058
Medically reviewed by Drugs.com. Last updated on Sep 16, 2024.
Drug Facts
Bisacodyl USP 10 mg
Stimulant Laxative
- for temporary relief of occasional constipation and irregularity
- this product generally produces bowel movement in 15 minutes to 1 hour
- For rectal use only
- stomach pain, nausea or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
When using this product it may cause abdominal discomfort, faintness, rectal burning and mild cramps
- you have rectal bleeding or no bowel movement after using this product. These could be signs of a serious condition.
- you need to use a laxative for more than 1 week
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
| adults and children 12 years of age and over | 1 suppository in a single daily dose. Peel open plastic wrapper. Insert suppository well into rectum, pointed end first. Retain about 15 to 20 minutes. |
| children 6 to under 12 years of age | 1/2 suppository in a single daily dose |
| children under 6 years of age | Do not use |
- store at 20-25°C (68-77°F)
- Lot No. & Exp. Date: see wrapper or box
hydrogenated vegetable oil
(800) 616-2471
Distributed by:
MAJOR
® PHARMACEUTICALS
17177 N Laurel Park Drive, Suite 233
Livonia, MI 48152 USA
MAJOR®
NDC 0904-5058-12
Biscolax™
Laxative
Doctor Recommended Ingredient
Fast Dependable Relief
Bisacodyl, USP 10 mg.
12 SUPPOSITORIES
Compare to active ingredient of
Dulcolax
® Suppositories*
| BISCOLAX LAXATIVE
bisacodyl suppository |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Major (191427277) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
