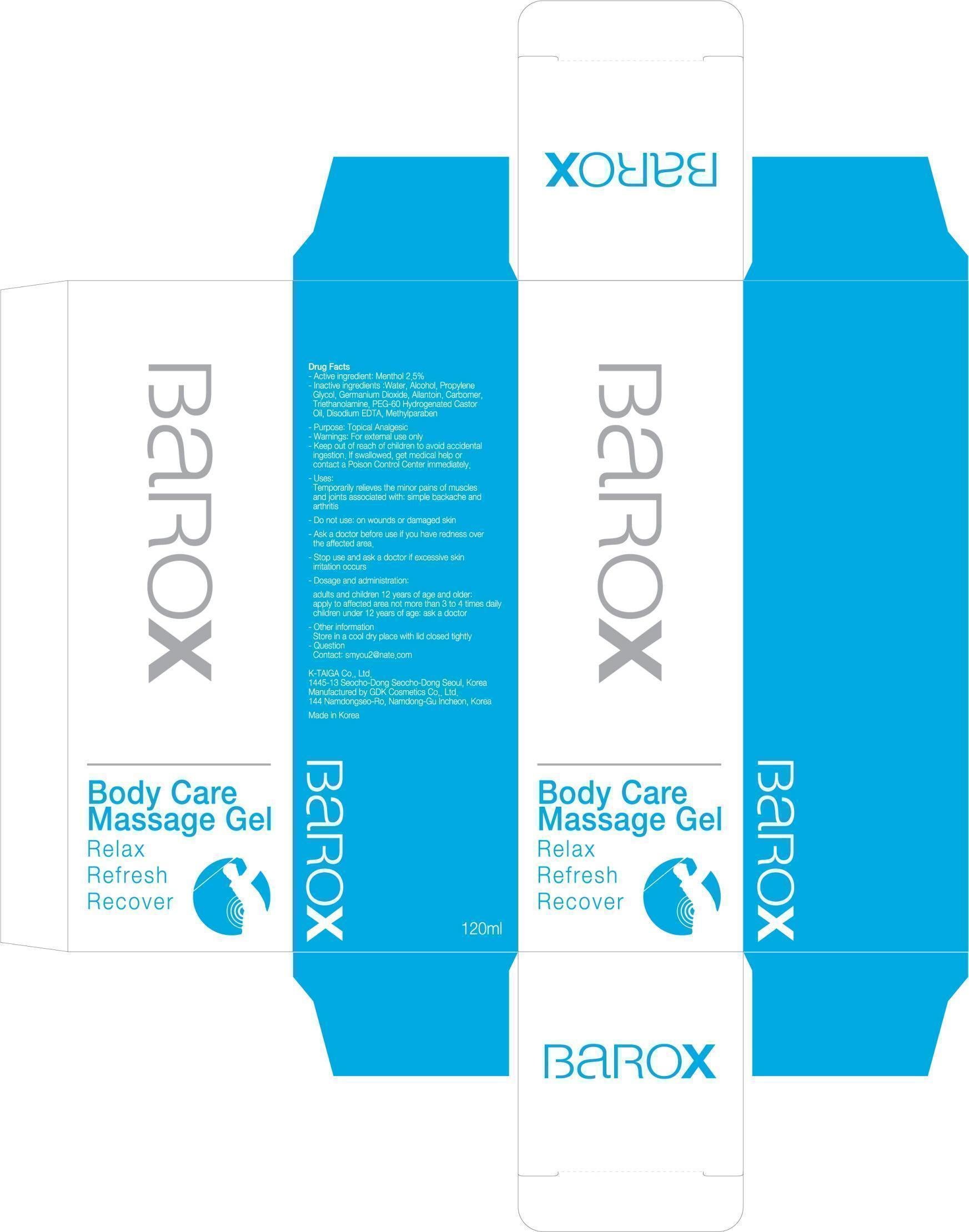
BAROX
Dosage form: gel
Ingredients: MENTHOL 3g in 120mL
Labeler: KTAIGA CO., LTD.
NDC code: 52227-100
Medically reviewed by Drugs.com. Last updated on Aug 5, 2025.
Active ingredient: Menthol 2.5%
Inactive ingredients:
Water, Alcohol, Propylene Glycol, Germanium Dioxide, Allantoin, Carbomer, Triethanolamine, PEG-60 Hydrogenated Castor Oil, Disodium EDTA, Methylparaben
Purpose: Topical Analgesic
Warnings: For external use only
Keep out of reach of children:
Keep out of reach of children to avoid accidental ingestion.
If swallowed, get medical help or contact a Poison Control Center immediately.
Uses:
Temporarily relieves the minor pains of muscles and joints associated with:
simple backache and arthritis
Dosage and administration:
adults and children 12 years of age and older: apply to affected area not more than 3 to 4 times daily
children under 12 years of age: ask a doctor
Ask a doctor before use if you have redness over the affected area.
Do not use: on wounds or damaged skin
Stop use and ask a doctor if excessive skin irritation occurs.
Other information:
Store in a cool dry place with lid closed tightly
Question
Contact: smyou2@nate.com
| BAROX
menthol gel |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| Labeler - KTAIGA CO., LTD. (557819324) |
| Registrant - KTAIGA CO., LTD. (557819324) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| KTAIGA CO., LTD. | 557819324 | manufacture(52227-100) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
