Bacitracin: Package Insert / Prescribing Info
Package insert / product label
Dosage form: ophthalmic ointment
Drug class: Ophthalmic anti-infectives
Medically reviewed by Drugs.com. Last updated on Nov 11, 2024.
On This Page
Bacitracin Description
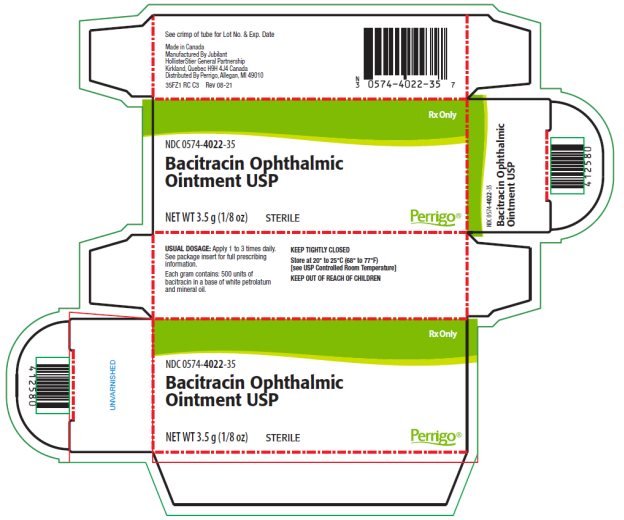
Each gram of ointment contains 500 units of Bacitracin in a low melting special base containing White Petrolatum and Mineral Oil.
Bacitracin - Clinical Pharmacology
The antibiotic, Bacitracin, exerts a profound action against many gram-positive pathogens, including the common Streptococci and Staphlococci. It is also destructive for certain gram-negative organisms. It is ineffective against fungi.
Indications and Usage for Bacitracin
For the treatment of superficial ocular infections involving the conjunctiva and/or cornea caused by Bacitracin susceptible organisms.
Contraindications
This product should not be used in patients with a history of hypersensitivity to Bacitracin.
Precautions
Bacitracin ophthalmic ointment should not be used in deep-seated ocular infections or in those that are likely to become systemic. The prolonged use of antibiotic containing preparations may result in overgrowth of nonsusceptible organisms particularly fungi. If new infections develop during treatment appropriate antibiotic or chemotherapy should be instituted.
Adverse Reactions/Side Effects
Bacitracin has such a low incidence of allergenicity that for all practical purposes side reactions are practically non-existent. However, if such reaction should occur, therapy should be discontinued.
To report SUSPECTED ADVERSE REACTIONS, contact Perrigo at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Related/similar drugs
Bacitracin Dosage and Administration
The ointment should be applied directly into the conjunctival sac 1 to 3 times daily. In blepharitis all scales and crusts should be carefully removed and the ointment then spread uniformly over the lid margins. Patients should be instructed to take appropriate measures to avoid gross contamination of the ointment when applying the ointment directly to the infected eye.
How is Bacitracin supplied
NDC 0574-4022-35 3.5 g (1/8 oz.) sterile tamper evident tubes with ophthalmic tip.
Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].
Made in Canada
Manufactured by Jubilant HollisterStier General Partnership
Kirkland, Quebec H9H 4J4 Canada
Distributed By Perrigo, Allegan, MI 49010
Minneapolis, MN 55427
35F00 RC J1
Rev 08-21
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 3.5 g Carton
Rx Only
NDC 0574-4022-35
Bacitracin Ophthalmic Ointment USP
NET WT 3.5 g (1/8 oz)
STERILE
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
| BACITRACIN
bacitracin ointment |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Padagis US LLC (967694121) |
More about bacitracin ophthalmic
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Side effects
- Drug class: ophthalmic anti-infectives
- Breastfeeding
- En español